|
|
|
|
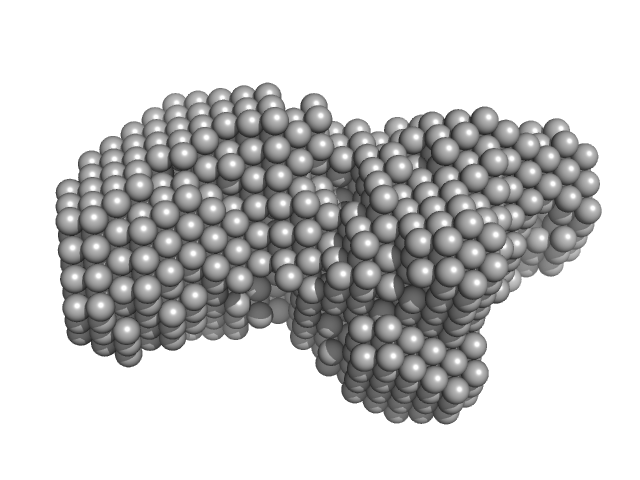
|
| Sample: |
Enhanced disease susceptibility monomer, 72 kDa Arabidopsis thaliana protein
|
| Buffer: |
50 mM NaCl, 50 mM HEPES, 1% glyercol, 1 mM DTT, pH: 8
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2015 May 19
|
Arabidopsis immunity regulator EDS1 in a PAD4/SAG101-unbound form is a monomer with an inherently inactive conformation.
J Struct Biol :107390 (2019)
Voss M, Toelzer C, Bhandari DD, Parker JE, Niefind K
|
| RgGuinier |
3.1 |
nm |
| Dmax |
10.5 |
nm |
| VolumePorod |
92 |
nm3 |
|
|
|
|
|
|
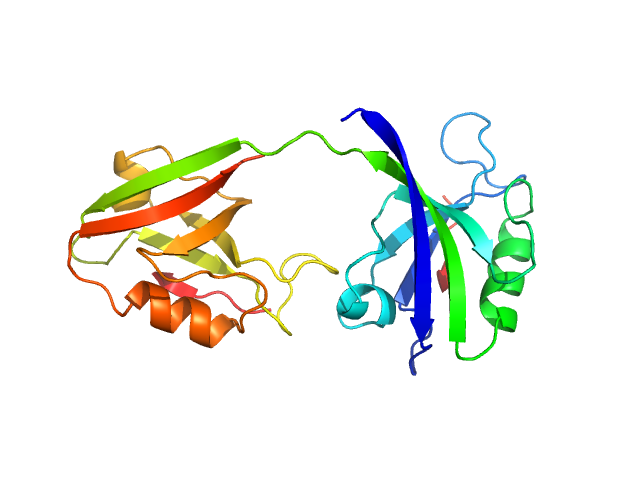
|
| Sample: |
PDZ1-2 fragment of PSD-95/Disks large homolog 4 monomer, 21 kDa Homo sapiens protein
|
| Buffer: |
20 mM TRIS/HCl, 150 mM NaCl + 10 mM RRESEI, pH: 8.5
|
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2015 Jun 11
|
The dual PDZ domain from Postsynaptic density protein 95 forms a scaffold with peptide ligand
Biophysical Journal (2020)
Rodzli N, Lockhart-Cairns M, Levy C, Chipperfield J, Bird L, Baldock C, Prince S
|
| RgGuinier |
3.0 |
nm |
| Dmax |
13.1 |
nm |
| VolumePorod |
32 |
nm3 |
|
|
|
|
|
|
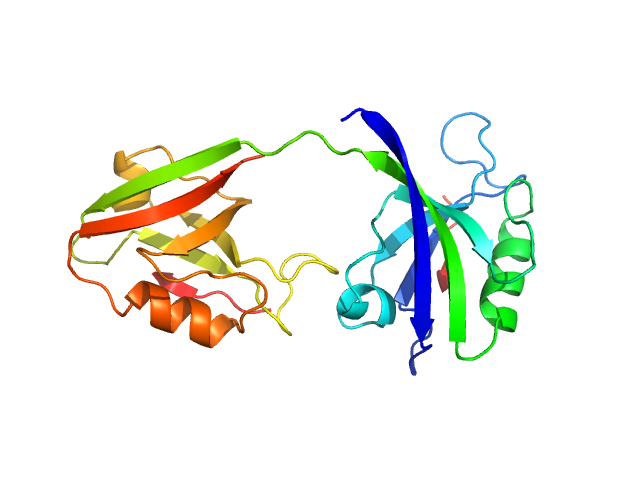
|
| Sample: |
PDZ1-2 fragment of PSD-95/Disks large homolog 4 monomer, 21 kDa Homo sapiens protein
|
| Buffer: |
20 mM TRIS/HCl, 150 mM NaCl + 5 mM RRESEI, pH: 8.5
|
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2015 Jun 11
|
The dual PDZ domain from Postsynaptic density protein 95 forms a scaffold with peptide ligand
Biophysical Journal (2020)
Rodzli N, Lockhart-Cairns M, Levy C, Chipperfield J, Bird L, Baldock C, Prince S
|
| RgGuinier |
2.6 |
nm |
| Dmax |
12.4 |
nm |
| VolumePorod |
32 |
nm3 |
|
|
|
|
|
|
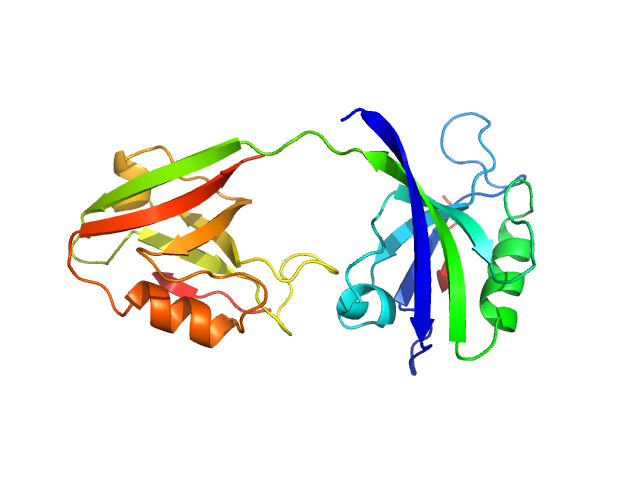
|
| Sample: |
PDZ1-2 fragment of PSD-95/Disks large homolog 4 monomer, 21 kDa Homo sapiens protein
|
| Buffer: |
20 mM TRIS/HCl, 150 mM NaCl + 10 mM RRESEI, pH: 8.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2016 Jan 26
|
The dual PDZ domain from Postsynaptic density protein 95 forms a scaffold with peptide ligand
Biophysical Journal (2020)
Rodzli N, Lockhart-Cairns M, Levy C, Chipperfield J, Bird L, Baldock C, Prince S
|
| RgGuinier |
2.3 |
nm |
| Dmax |
6.8 |
nm |
| VolumePorod |
28 |
nm3 |
|
|
|
|
|
|
|
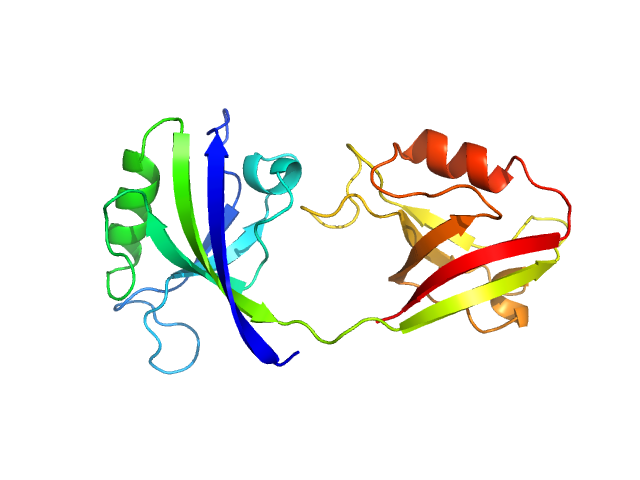
| Sample: |
PDZ1-2 fragment of PSD-95/Disks large homolog 4 monomer, 21 kDa Homo sapiens protein
|
| Buffer: |
20 mM TRIS/HCl, 150 mM NaCl, pH: 8.5
|
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2015 Jun 11
|
The dual PDZ domain from Postsynaptic density protein 95 forms a scaffold with peptide ligand
Biophysical Journal (2020)
Rodzli N, Lockhart-Cairns M, Levy C, Chipperfield J, Bird L, Baldock C, Prince S
|
| RgGuinier |
2.4 |
nm |
| Dmax |
9.2 |
nm |
| VolumePorod |
32 |
nm3 |
|
|
|
|
|
|
|
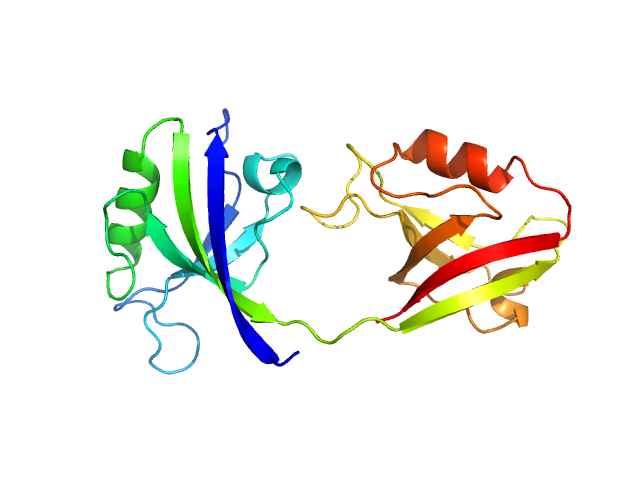
| Sample: |
PDZ1-2 fragment of PSD-95/Disks large homolog 4 monomer, 21 kDa Homo sapiens protein
|
| Buffer: |
20 mM TRIS/HCl, 150 mM NaCl, pH: 8.5
|
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2015 Jun 11
|
The dual PDZ domain from Postsynaptic density protein 95 forms a scaffold with peptide ligand
Biophysical Journal (2020)
Rodzli N, Lockhart-Cairns M, Levy C, Chipperfield J, Bird L, Baldock C, Prince S
|
| RgGuinier |
2.5 |
nm |
| Dmax |
9.5 |
nm |
| VolumePorod |
32 |
nm3 |
|
|
|
|
|
|
|
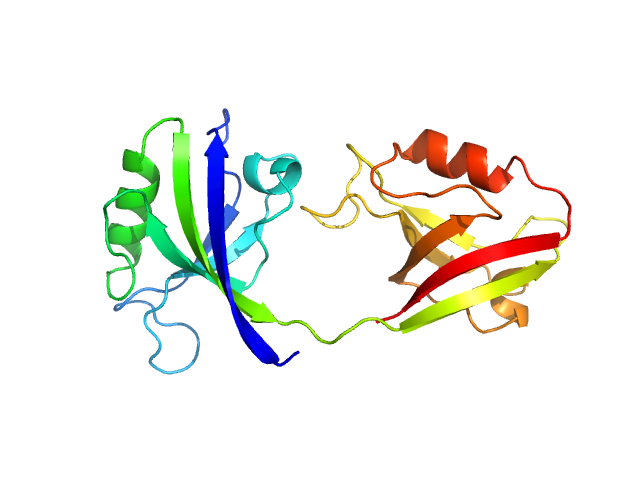
| Sample: |
PDZ1-2 fragment of PSD-95/Disks large homolog 4 monomer, 21 kDa Homo sapiens protein
|
| Buffer: |
20 mM TRIS/HCl, 150 mM NaCl, pH: 8.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2016 Jan 26
|
The dual PDZ domain from Postsynaptic density protein 95 forms a scaffold with peptide ligand
Biophysical Journal (2020)
Rodzli N, Lockhart-Cairns M, Levy C, Chipperfield J, Bird L, Baldock C, Prince S
|
| RgGuinier |
2.4 |
nm |
| Dmax |
6.4 |
nm |
| VolumePorod |
31 |
nm3 |
|
|
|
|
|
|
|
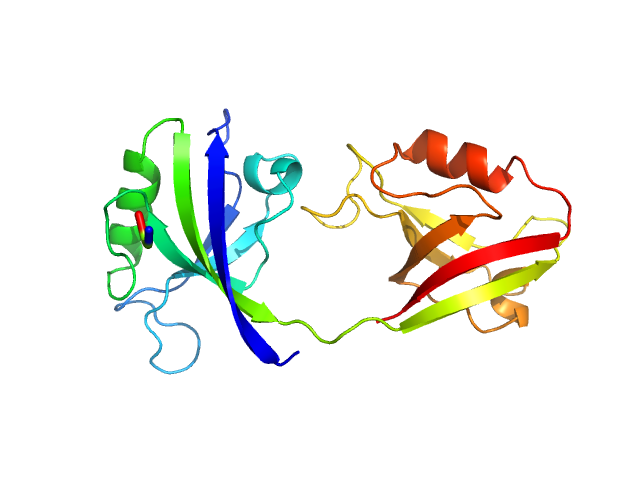
| Sample: |
PDZ1-2 fragment of PSD-95/Disks large homolog 4 monomer, 21 kDa Homo sapiens protein
|
| Buffer: |
20 mM TRIS/HCl, 150 mM NaCl + 10 mM GSH, pH: 8.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2016 Sep 16
|
The dual PDZ domain from Postsynaptic density protein 95 forms a scaffold with peptide ligand
Biophysical Journal (2020)
Rodzli N, Lockhart-Cairns M, Levy C, Chipperfield J, Bird L, Baldock C, Prince S
|
| RgGuinier |
2.7 |
nm |
| Dmax |
6.3 |
nm |
| VolumePorod |
29 |
nm3 |
|
|
|
|
|
|
|
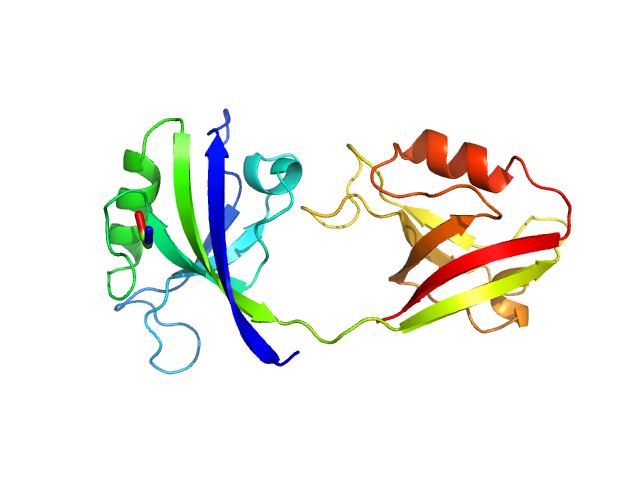
| Sample: |
PDZ1-2 fragment of PSD-95/Disks large homolog 4 monomer, 21 kDa Homo sapiens protein
|
| Buffer: |
20 mM TRIS/HCl, 150 mM NaCl + 16 mM GSH, pH: 8.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2016 Sep 16
|
The dual PDZ domain from Postsynaptic density protein 95 forms a scaffold with peptide ligand
Biophysical Journal (2020)
Rodzli N, Lockhart-Cairns M, Levy C, Chipperfield J, Bird L, Baldock C, Prince S
|
| RgGuinier |
3.1 |
nm |
| Dmax |
7.0 |
nm |
| VolumePorod |
41 |
nm3 |
|
|
|
|
|
|
|
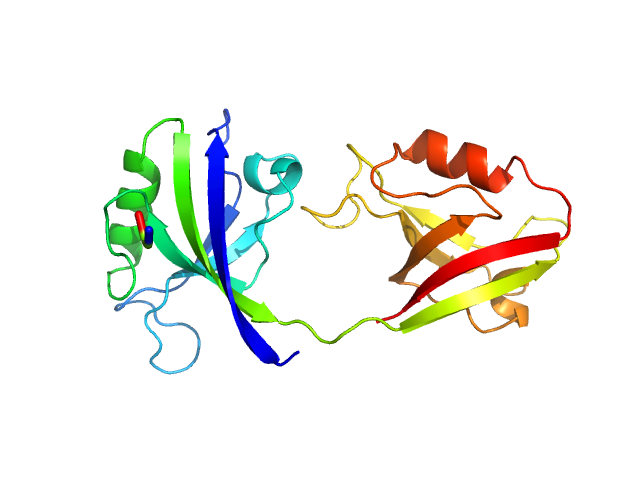
| Sample: |
PDZ1-2 fragment of PSD-95/Disks large homolog 4 monomer, 21 kDa Homo sapiens protein
|
| Buffer: |
20 mM TRIS/HCl, 150 mM NaCl + 10 mM GSH, pH: 8.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2016 Jan 26
|
The dual PDZ domain from Postsynaptic density protein 95 forms a scaffold with peptide ligand
Biophysical Journal (2020)
Rodzli N, Lockhart-Cairns M, Levy C, Chipperfield J, Bird L, Baldock C, Prince S
|
| RgGuinier |
2.4 |
nm |
| Dmax |
7.2 |
nm |
| VolumePorod |
30 |
nm3 |
|
|