|
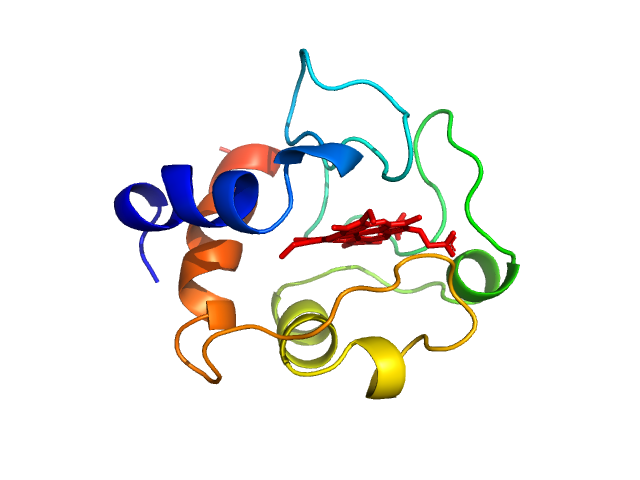
Synchrotron SAXS data from solutions of cytochrome c from equine heart in 25 mM HEPES, 100 mM NaCl, 3% v/v glycerol, pH 7.5 were collected on the EMBL P12 beam line at the PETRA III storage ring (Hamburg, Germany) using a Pilatus 6M detector at a sample-detector distance of 3 m and at a wavelength of λ = 0.124 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). In-line size exclusion chromatography (SEC) SAXS was employed. The SEC parameters were as follows: A 35 μl sample at 10 mg/ml was injected at room temperature onto a GE Healthcare Superdex 200 Increase 5/150 column and the sample was eluted at a flow rate of 0.4 mL/min. 58 successive 0.5 second frames were collected through the SEC-elution peak. The data were normalized to the intensity of the transmitted beam and radially averaged and the scattering of an appropriate the solvent-blank was subtracted and the data scaled and averaged to produce the final SAXS profile displayed in this entry. CHROMIXS was used to assess the stability of the radius of gyration through the SEC peak while the quoted experimental MW was determined using SEC-MALLS/RI measurements performed in parallel to the SAXS data collection (additional Rg and MW correlation data are included in the full entry zip archive and all individual unsubtracted SEC-SAXS data frames).
SEC column = UNKNOWN. Sample injection volume = UNKNOWN. Flow rate = UNKNOWN
|
|
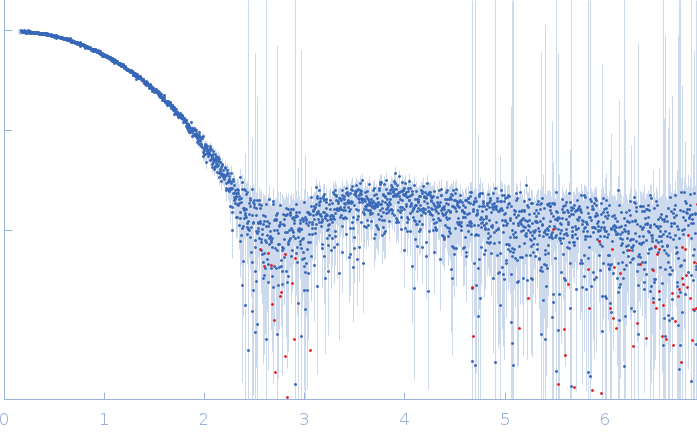 s, nm-1
s, nm-1