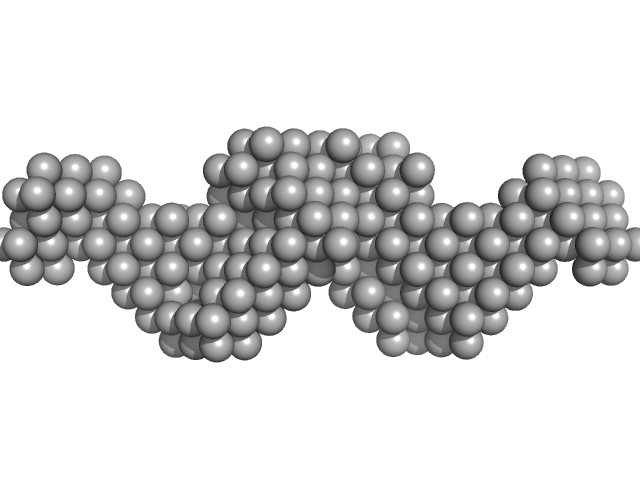
|
X-ray synchrotron radiation scattering data from solutions of Leucine-rich repeat transmembrane neuronal protein 2, LRRTM2 in 20 mM Tris 150 mM NaCl, 3% glycerol were collected on the ID14-3 beam line at the ESRF storage ring (Grenoble, France) using a 2D Photon counting Pilatus 1M pixel detector (s = 4π sin θ/λ, where 2θ is the scattering angle). Different solute concentrations in the range 1.30-3.40 mg/ml were measured. 10 successive 1 second frames were collected at a sample temperature of 10 degrees centigrade. SAXS data from the samples and corresponding matched solvent blank were normalized to the intensity of the transmitted beam and radially averaged and the scattering of the solvent-blank was subtracted. The SAXS data for this entry were derived from the concentration series extrapolated to infinite dilution.
Cell temperature = UNKNOWN
|
|
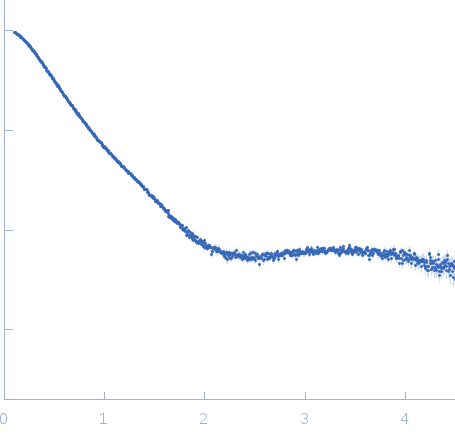 s, nm-1
s, nm-1