|
Synchrotron SAXS data from solutions of bovine serum albumin in 25 mM MOPS, 250 mM NaCl, 50 mM KCl, 2 mM TCEP, 0.1% NaN3, pH 7.5 were collected on the SAXS/WAXS beam line at Australian Synchrotron (Melbourne, Australia) using a Pilatus 1M detector at a sample-detector distance of 2.7 m and at a wavelength of λ = 0.10332 nm (I(s) vs s, where s = 4πsinθ/λ and 2θ is the scattering angle). Scattering data are on an absolute scale (cm-1) set by reference to the scattering from pure H2O. I(0) values must be corrected for the shear flow sample cell by a 2.05 multiplicative factor. Data were acquired in SEC-SAXS mode, summed from 10 measurement frames where Rg was flat and at a maximum. The average concentration over the frames was 0.57 mg/mL, spanning values from 0.31 - 0.76 mg/mL. Sample concentrations were measured by A280 (extinction coefficient for 1 mg/mL, 10 mm pathlength; E0.1% = 0.646), corrected for the 3.1 mm path-length of the UV measurement cell. MULCh was used to calculate the partial specific volume (0.0.732 mL/g) and X-ray contrast (Δρ = 2.863e10 cm-2). The protein is a monmer in the solution conditions measured. The Multi-state (Multi-FoXS) fits to the data assumed flexible residues 183-187 and 381-384 in the loops that link the two tightly networked domains and proposed to be responsible for domain movements based upon their high temperature factors in the crystal structure.
Storage temperature = UNKNOWN
|
|
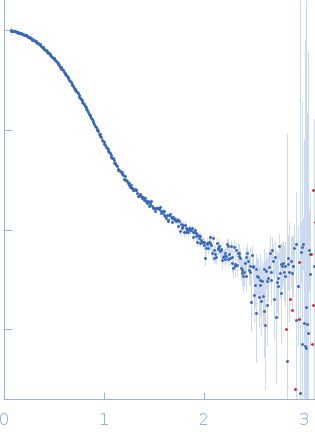 s, nm-1
s, nm-1