|
Size-exclusion chromatography SAXS (SEC-SAXS) data from solutions of Synechocystis fluorescence recovery protein SynFRP.8-109 in 20 mM Tris-HCl, 150 mM NaCl, 0.1 mM EDTA, 2 mM dithiothreitol, 3 % v/v glycerol, pH 7.6 were collected at the EMBL-P12 bioSAXS beam line at the PETRA III storage ring (Hamburg, Germany) using a Pilatus 2M detector at a wavelength of λ = 0.124 nm (I(s) vs s, where s = 4πsinθ/λ and 2θ is the scattering angle). 100 μl of sample at 5.00 mg/ml were loaded on a Superdex 200 Increase 10/300 column (GE Healthcare) at a flow rate of 0.5 ml/min. 3600 successive 1 second frames were collected from the column eluate that were additionally analyzed using multi-angle laser light scattering (MALLS), refractive index (RI) and and dynamic light scattering (DLS) measurements. The SAXS data were processed using the software CHROMIXS as part of the ATSAS 2.8.3 SAS data analysis package.
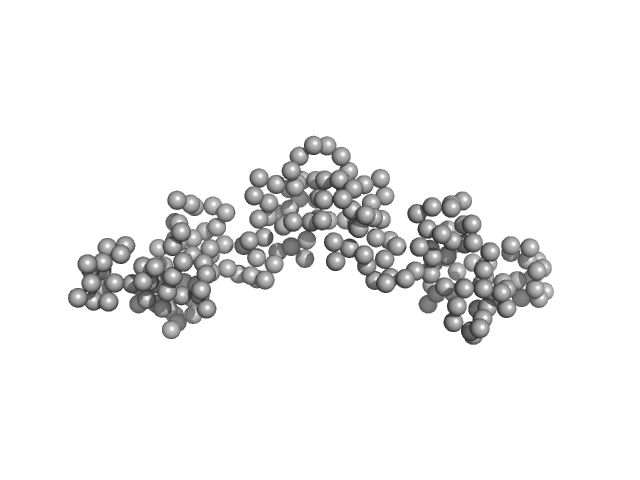
The experimental MW quoted above is derived from the average value obtained from MALLS (28 kda), MW from Porod volume (23 kDa), MW from the volume of correlation (23 kDa) and from the method of Fischer et al, SAXSMOW (30 kDa). The hydrodynamic radius obtained from DLS is 2.86 nm. Additional GASBOR models, with spatial alignments and the NSD estimate can be located in the full entry zip archive.
|
|
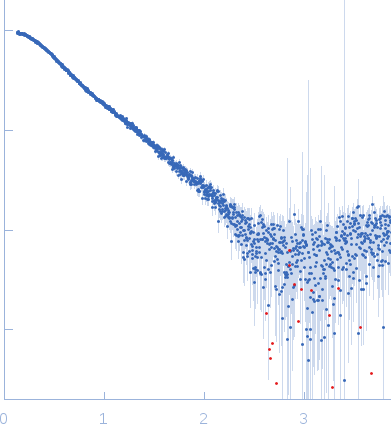 s, nm-1
s, nm-1