|
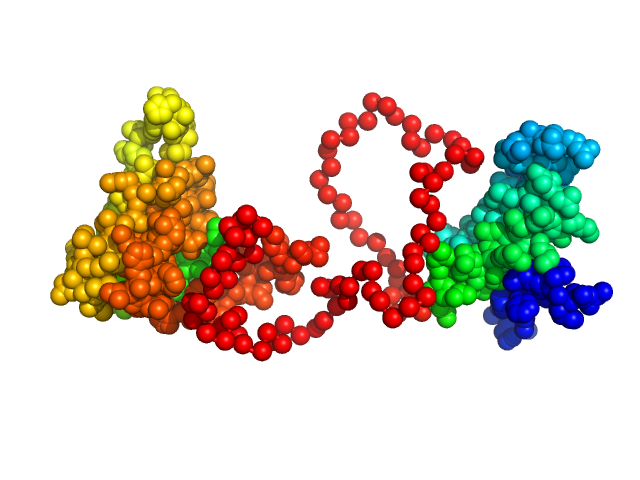
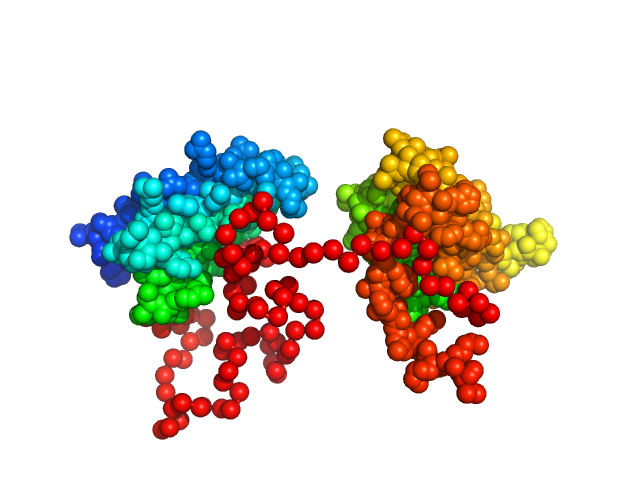
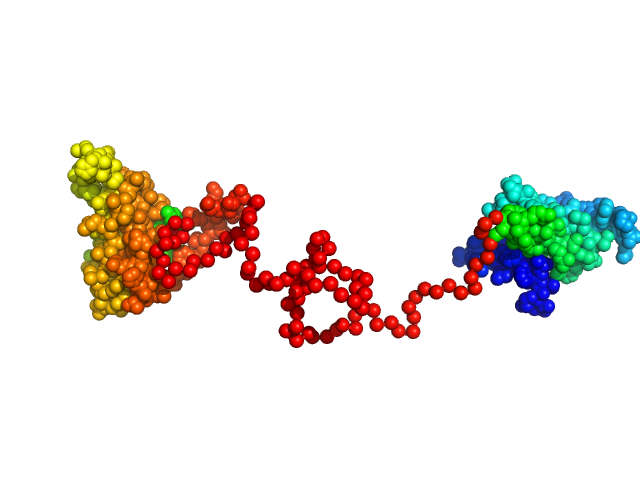
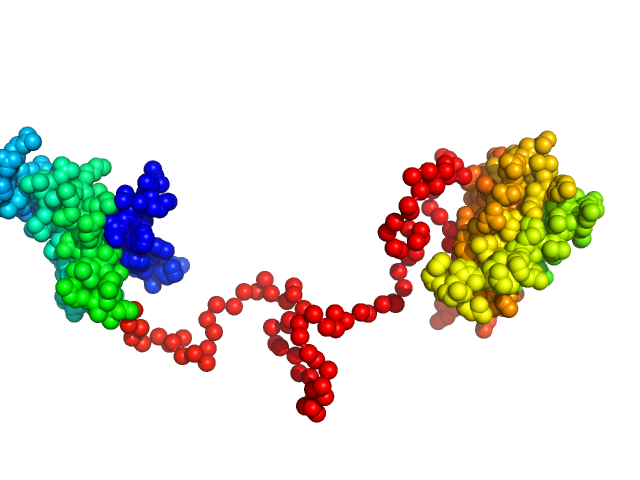
Synchrotron SAXS data from solutions of the dsRBD1 and dsRBD2 domains of Drosophila helicase dosage compensation regulator, MLE, in 20 mM NaPO4, 200 mM NaCl, 1 mM DTT, pH 6.5 were collected on the BM29 beam line at the ESRF (Grenoble, France) using a Pilatus 1M detector at a sample-detector distance of 2.9 m and at a wavelength of λ = 0.09919 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). A solute concentration of 5 mg/ml was measured at 20°C. 10 successive 1 second frames were collected per sample (three replicates total). The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
Construct contains an additional two amino acids (Gly-Ala) at the N-terminus as a remaining product the TEV-protease cleavage.
The data displayed in this entry consists of merged data from three replicates of equal sample concentration (5.0 mg/ml). Thus, an effective total of 30 frames x 1s exposures is collated for this curve. The data were merged using Primus and datmerge of the ATSAS 2.8.4-1 package maintained by SBGrid.
|
|
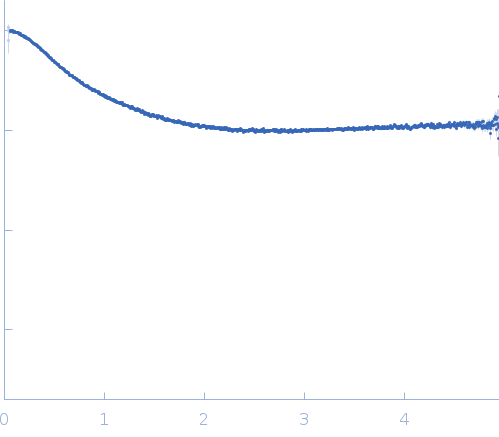 s, nm-1
s, nm-1
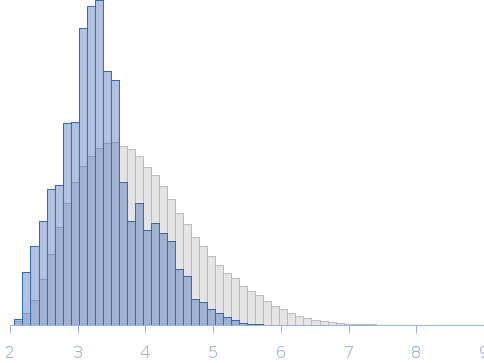 Rg, nm
Rg, nm