|
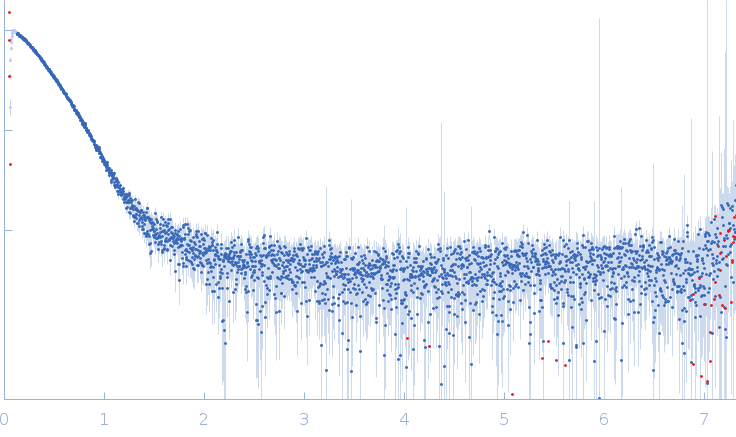
Synchrotron SAXS data from the 1:2 heterotrimer of pUL7 and pUL51 from herpes simplex virus 1 in 20 mM HEPES, 200 mM NaCl, 3% (v/v) glycerol, 1 mM DTT, pH 7.5 were collected on the EMBL P12 beam line at PETRA III (Hamburg, Germany) using a Pilatus 6M detector at a sample-detector distance of 3 m and at a wavelength of λ = 0.124 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). In-line size-exclusion chromatography (SEC) SAS was employed. The SEC parameters were as follows: A 90.00 μl sample at 8 mg/ml was injected at a 0.50 ml/min flow rate onto a GE Superdex 200 Increase 10/300 column at 20.2°C. 35 successive 0.995 second frames were collected through the major SEC-elution peak (corresponding to the heterohexamer) and processed using CHROMIXS. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
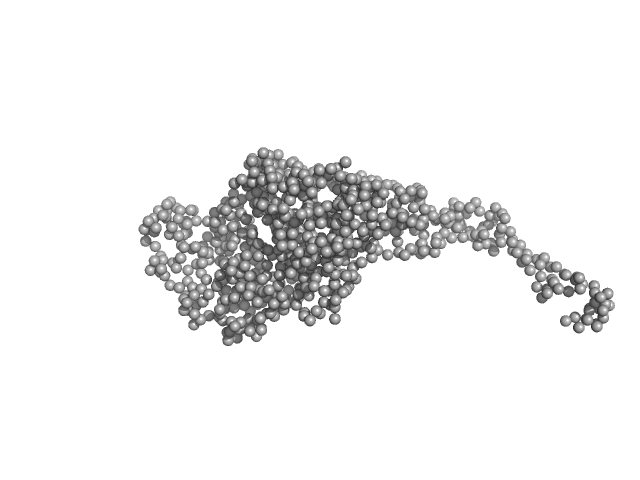
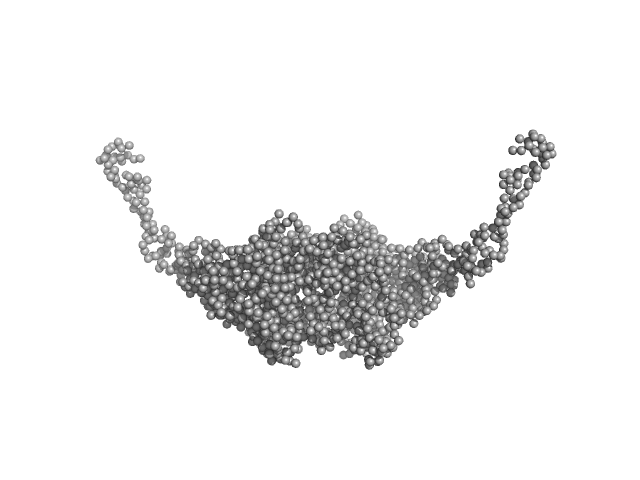
The quoted experimental molecular mass, that corresponds to a 1:2 pUL7:pUL51 heterotrimer, was determined from SEC-MALLS-RI measurements performed in parallel to the SEC-SAXS. The SEC-SAXS data, Rg correlations through the heterotrimer elution peak as well as the the SEC-MALLS-RI data are made available in the full-entry zip archive. The GASBORMX model displayed in this entry represents an 80 % volume fraction of the heterotrimer (corresponding fit, top) combined with a 20% volume fraction of a 2:4 pUL7:pUL51 heterohexamer (corresponding fit, bottom), due to incomplete SEC-separation between the two forms of the complex (also refer to SASBDB entry SASDG47).
|
|
 s, nm-1
s, nm-1