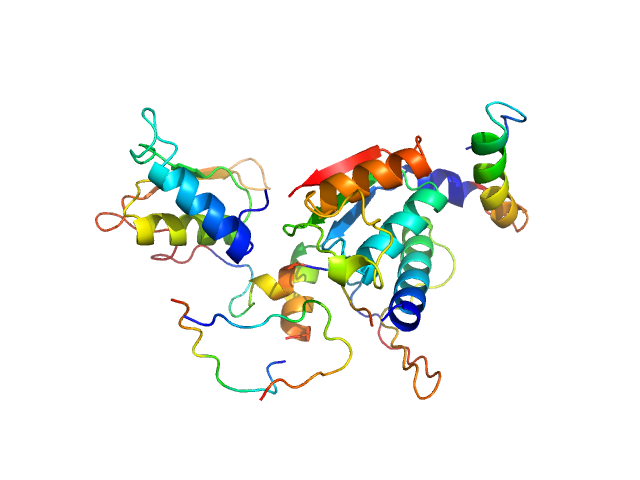
| MWI(0) | 48 | kDa |
| MWexpected | 68 | kDa |
| VPorod | 74 | nm3 |
|
log I(s)
2.80×10-3
2.80×10-4
2.80×10-5
2.80×10-6
|
 s, nm-1
s, nm-1
|
|
|
|
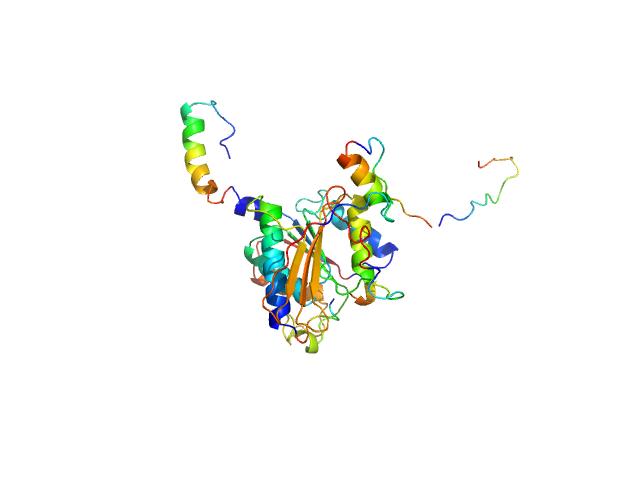

|
|
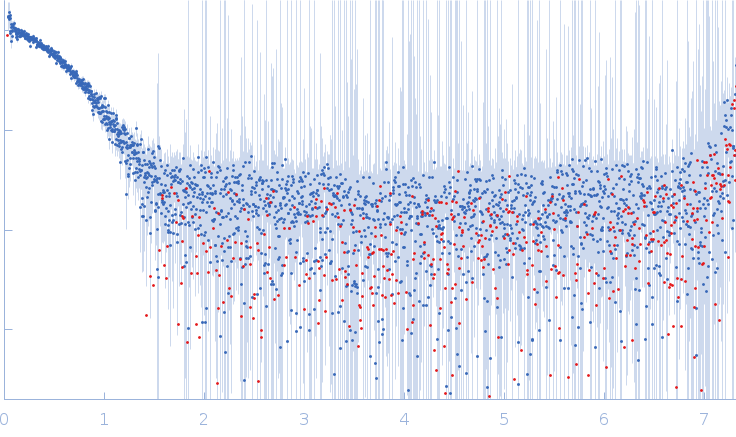
Synchrotron SAXS
data from solutions of
Ubiquitin activating enzyme 5 (0.3 mg/ml)
in
20 mM Tris, 150 mM NaCl, 2 mM DTT, pH 7.5
were collected
on the
EMBL P12 beam line
at the PETRA III storage ring
(DESY; Hamburg, Germany)
using a Pilatus 6M detector
at a sample-detector distance of 3 m and
at a wavelength of λ = 0.123982 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
One solute concentration of 0.30 mg/ml was measured
at 15°C.
50 successive
0.045 second frames were collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
|
|
|||||||||||||||||||||||||||||||||