|
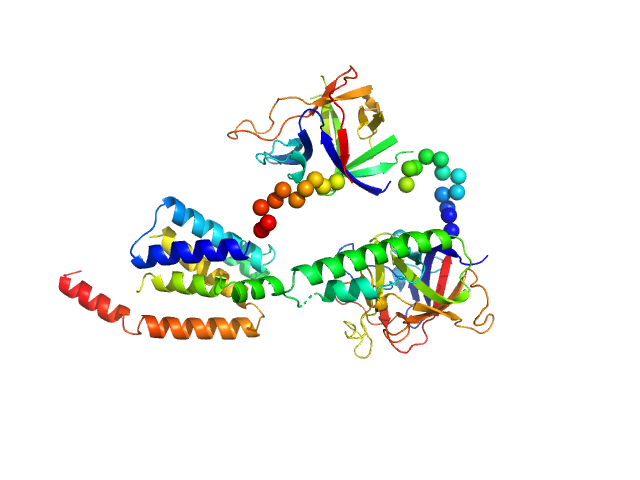
X-ray scattering data from the from the N-terminal domains of the inositol 1,4,5-triphosphate receptor type I (IP3R) in 15 mM Tris-HCl 300 mM NaCl 1 mM TCEP 5 mM EGTA pH 8.0 were collected on a Bruker Nanostar instrument at the Bragg Institute (Australian Nuclear Science and Technology Organisation, Lucas Heights, Australia) using a HiStar 2D detector (I(s) vs s, where s = 4π sin θ/λ and 2θ is the scattering angle; λ=0.15406 nm). Approximately 15 µL of a 2.5 mg/ml protein solution was loaded into a quartz capillary mounted in a stainless steel holder. A single 3600s second frame was collected, and the buffer was collected in an analogous fashion. The data were normalized to the intensity of the transmitted beam, radially averaged, and the scattering of the solvent-blank was subtracted. The data are on an arbitrary scale, and the mass of the protein was determined using a lysozyme secondary standard at a concentration of 5.5 mg/ml. The model and corresponding fits include are derived from a rigid body model using BUNCH05.
Model derived from PDB structures 1XZZ and 1N4K (which is missing many residues).
|
|
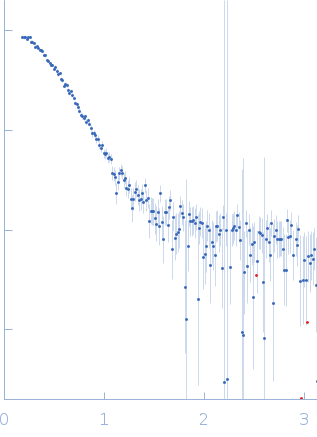 s, nm-1
s, nm-1