| MWexperimental | 21 | kDa |
| MWexpected | 21 | kDa |
| VPorod | 27 | nm3 |
|
log I(s)
1.09×100
1.09×10-1
1.09×10-2
1.09×10-3
|
 s, nm-1
s, nm-1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
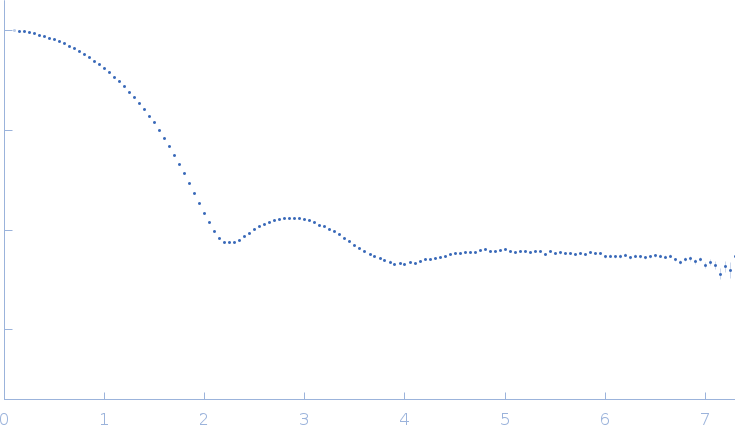
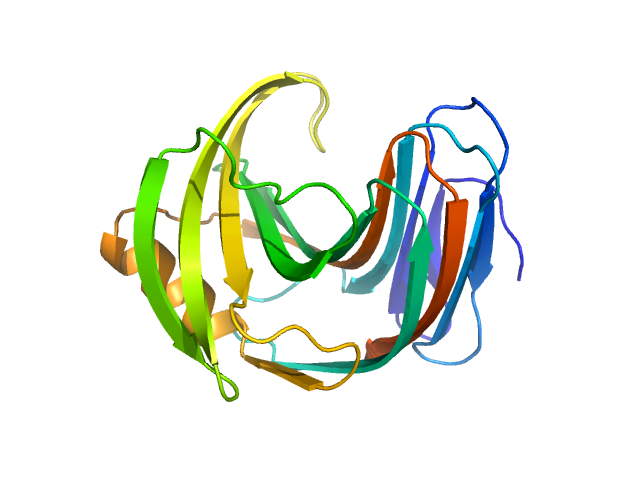
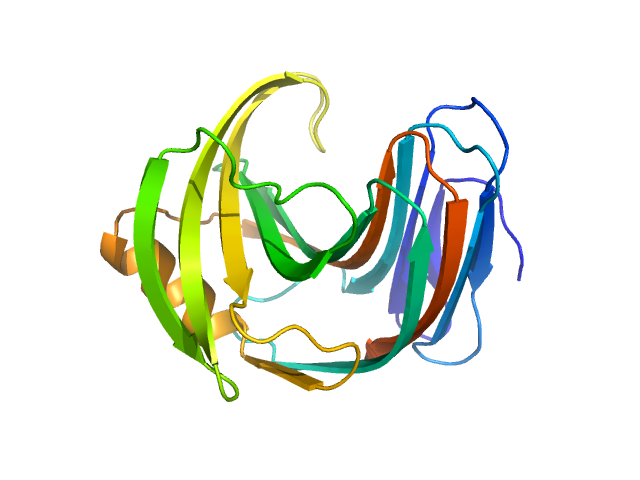
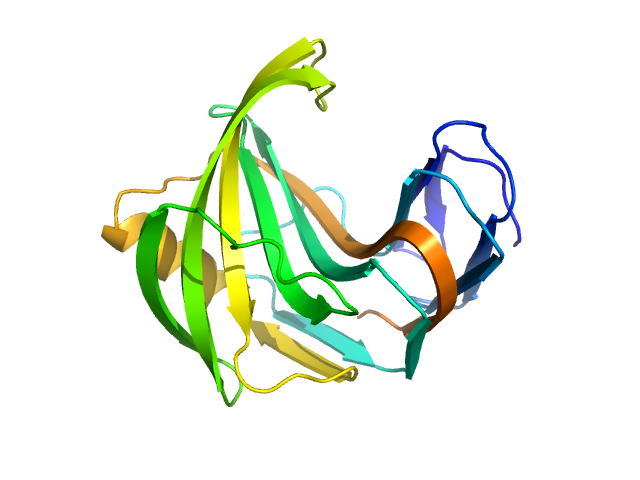
The consensus SAXS profile for xylanase was generated by the datcombine tool (ATSAS 3.1.0) with both outlier- and error-filters applied. The data input to datcombine were four scattering profiles made up of pure SEC-SAXS (2), and merged SEC-SAXS-batch SAXS (2) data. All contributing data were independent measurements, and no individual measurement was represented more than once in the contributing scattering profile set. The buffer for substantial majority of the contributing data was 50 mM Tris, pH 7.5, 100 mM NaCl. Protein concentrations for batch measurements ranged from 6 - 14 mg/mL, and all batch data showed evidence of dimerization and had to be merged with SEC-SAXS measurements to remove any influence from the dimers. The xylanase atomistic model for CRYSOL, Pepsi-SAXS, and FoXS calculations was the PDB ID 2DFC with small-molecule crystallisation agents removed. Custom WAXSiS calculations (with Gromacs software) used the same coordinates and added explicit waters and appropriate number of ions for the MD calculations.
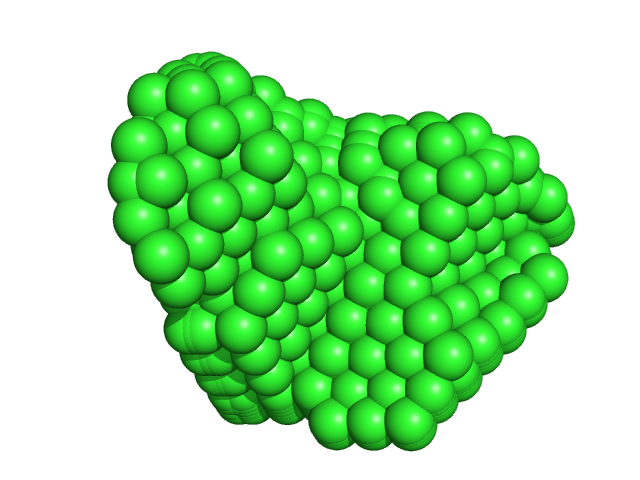
The data input to datcombine are made available for download in the associated zip file. Model fits are shown in order (top to bottom): DAMMIN, CRYSOL, Pepsi-SAXS, FoXS, and custom WAXSiS. The unusually good statistics for the consensus SAXS data generally give rise to large χ-square values for the model fits.
Tags:
benchmark
|
|
|||||||||||||||||||||||||||||||||||||||