|
Synchrotron SAXS data from solutions of wild type Pcs60p in 50 mM Hepes, 150 mM NaCl, 0.5 mM TCEP, pH 7.5 were collected on the EMBL P12 beam line at PETRA III (DESY, Hamburg, Germany) using a Pilatus 6M detector at a sample-detector distance of 3 m and at a wavelength of λ = 0.124 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 0.50 mg/ml was measured at 10°C. 29 successive 0.095 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
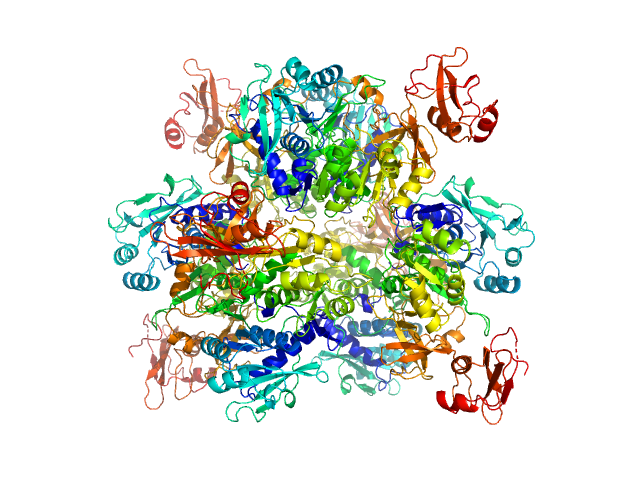
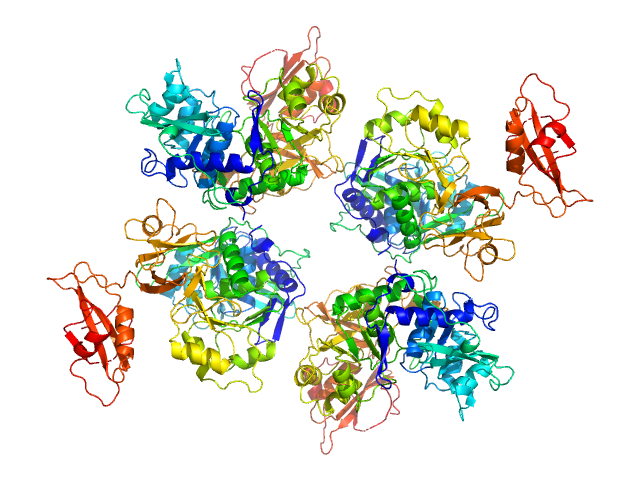
At 0.5 mg/ml, the approximate volume fraction of hexamer is 0.18 and tetramer, 0.82.
|
|
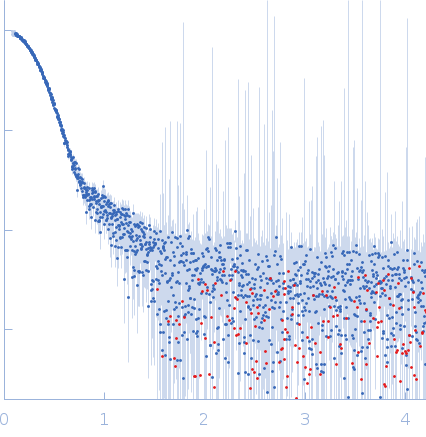 s, nm-1
s, nm-1