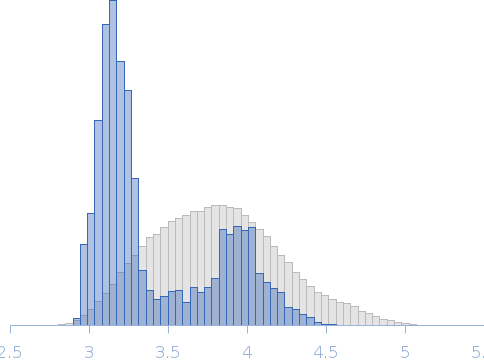
A Spring-Loaded Mechanism Governs the Clamp-like Dynamics of the Skp Chaperone.
Holdbrook DA, Burmann BM, Huber RG, Petoukhov MV, Svergun DI, Hiller S, Bond PJStructure 25(7):1079-1088.e3 (2017 Jul 5)
|
PMID: 28648612
doi: 10.1016/j.str.2017.05.018 |
Submitted to SASBDB: 2016 Jul 24 Published in SASBDB: |
|
|||||||||||||||||||||||