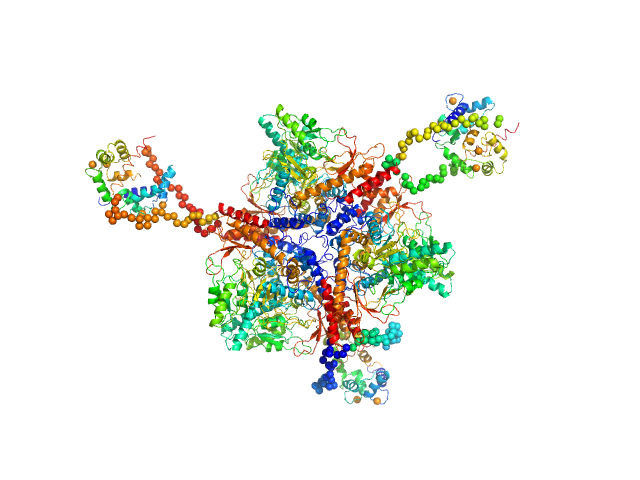
| MWexperimental | 400 | kDa |
| MWexpected | 357 | kDa |
| VPorod | 630 | nm3 |
|
log I(s)
8.76×104
8.76×103
8.76×102
8.76×101
|
 s, nm-1
s, nm-1
|
|
|
|
|
|
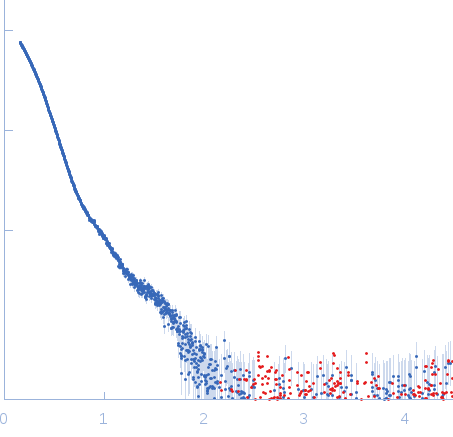
Synchrotron SAXS
data from solutions of
6:3 complex of GAD:CaM
in
50 mM HEPES 50 mM KCl, pH 7.5
were collected
on the
EMBL X33 beam line
at the DORIS III, DESY storage ring
(Hamburg, Germany)
using a MAR 345 Image Plate detector
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
Wavelength = UNKNOWN. Cell temperature = UNKNOWN. Storage temperature = UNKNOWN. Sample detector distance = UNKNOWN. X-ray Exposure time = UNKNOWN. Number of frames = UNKNOWN. Concentration = UNKNOWN
Tags:
X33
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||