|
Synchrotron SAXS data from solutions of Inactivated complement factor 1 (C1) in 50 mM EPPS, 145 mM NaCl, 3 mM CaCl2, pH 8.5, were collected using size-exclusion chromatography (SEC) SAXS on the EMBL-P12 bioSAXS beam line at the PETRAIII storage ring (Hamburg, Germany) equipped with a Pilatus 2M detector (I(s) vs s, where s = 4π sin θ/λ; 2θ is the scattering angle; λ = 0.124 nm). 100 successive 1 second frames were collected through the C1 SEC elution peak. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank (measured from macromolecular-free SEC running buffer) was subtracted and the resulting 1D profiles were scaled relative to each other and averaged to produce the final C1 SAXS data displayed in this entry.
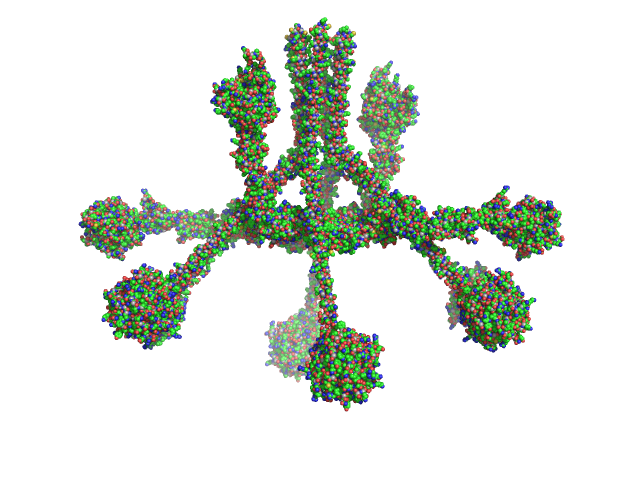
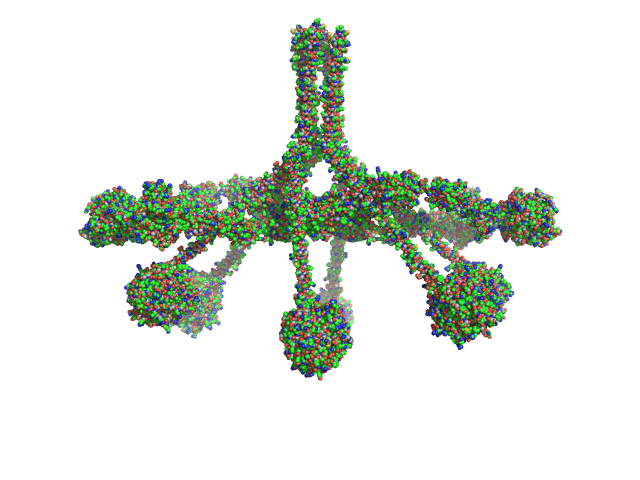
The expected MW quoted above (733 kDa) is calculated from the amino acid sequence of the oligomeric complex. The experimental MW of 790 kDa takes into account additional post-translational modifications of the protein. The models displayed in this entry - sequentially from top to bottom - represent those presented in Figure 4c of the main text and Supplementary Figures 2e and 4b, respectively, of Mortensen et al., 2017 Proc Natl Acad Sci U S A 114(5):986-991 .
|
|
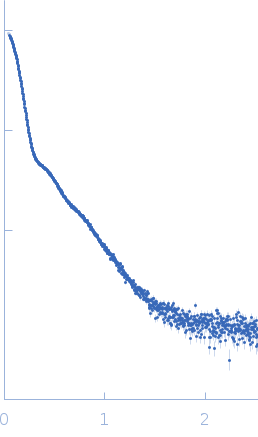 s, nm-1
s, nm-1