|
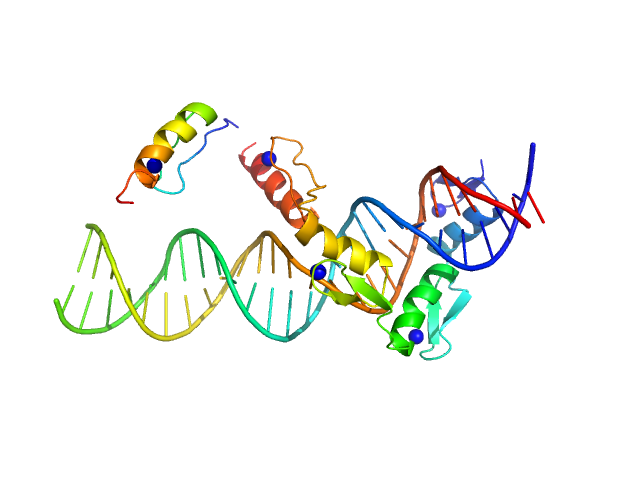
SAXS data were acquired from solutions of a 1:1 complex between the C-terminal zinc fingers (6-10) of the zinc finger and BTB domain-containing protein 38 (ZBTB38(1006-1153)) and the methylated C-terminal ZBTB38 binding sequence (mCZ38BS) in 10 mM Tris (pH 6.8), 1 mM tris(2-carboxy-ethyl)phosphine (TCEP), 0.05% NaN3 and10% D2O. An in-house Anton Paar SAXSess equipped with a mythen microstrip detector and set with a 10 mm line focus on sample enabled data to be taken immediately after assessment by NMR, which was crucial for characterizing the system within the lifetime of the sample. The data were desmeared by multiplication point by point by the ratio of the desmeared to smeared model P(r) profiles calculated using GNOM with a parameterised slit correction (AH = 2.9 nm-1, LH = 1.5 nm-1) and checked for accuracy against the Anton Paar SAXSQuant desmearing routine based on the Lake algorithm. Scattering data are on an absolute scale (cm-1) set by reference to the scattering from pure H2O. The concentration of the complex was estimated at 3.4 mg/mL, but the error is large as the complex was prepared using a concentration step after combining components and concentration estimated by change in volume which results in a significant error in the molecular mass determination from I(0) (30%). However, the Porod volume from the scattering data indicates the molecular mass of the scattering particle is within 3% of that expected for a 1:1 stoichiometry ZBTB38(1006-1153):mCZ38BS. MULCh was used to calculate the average partial specific volume (0.657 mL/g) and X-ray contrast (Δρ = 4.12e10 cm-2). Two phase (protein and DNA) modelling using MONSA, with simultaneous fitting of the ZBTB38(1006-1153):mCZ38BS complex and free mCZ38BS data, show the protein binds at one end of the duplex DNA. Initial attempts to generate an atomistic model used a homology model for the five C-terminal ZBTB38 ZFs built within SWISS-MODEL using ZFs 2-6 from the protein Aart in complex with DNA (PDB: 2I13) as the template. A model for CZ38BS was built in w3DNA after which the 3D-DART web server was utilized to introduce a widening of the major groove at the core ATCGGCG recognition motif and to introduce a 20° bend between residues 12-27. Docking of the ZBTB38 ZFs onto the CZ38BS was performed using the HADDOCK web server (version 2.2). The position of ZF10 (residues 1125-1153) in this model was uncertain, and so two sets of rigid body calculations were performed against the SAXS data; one with no protein:DNA constraints specified, and one including weak 10 Å distance constraints that maintained ZF10 in position to interact at the end of the DNA (between the phosphates of G1-A3 and the Cα of Lys1146, and the phosphates of G55-G57 and the Cα of Lys1145). From these calculations, there was no real consensus on the exact positioning of ZF10. The HADDOCK-generated atomistic model deposited here with its CRYSOL fit was obtained from SASREF including protein-DNA contact constraints involving ZF10. More recently we solved the X-ray crystal structure of ZBTB38 zinc fingers (ZFs) 6-9 in complex with an 18mer DNA sequence containing the core ATmCGGmCG mCZ38BS recognition motif (PDB: 6E93), revealing a reverse orientation of the zinc fingers with respect to the DNA compared to the original HADDOCK-generated model. An improved model has now been generated using the crystal structure (see “New Model Description: 01212020” in the full entry.) SASREF was again used to position ZF10. χ2 values obtained were somewhat improved: 1.65 versus 1.74 for data fits to the initial HADDOCK-generated model and with the corresponding CorMAP analysis giving similar P-value (0.029) using the latest version of CRYSOL in PRIMUSqt 3.0. As was the case for the HADDOCK-generated model, this improved model: i) agrees reasonably with the MONSA model, ii) the positioning of ZF10 remains uncertain and iii) ensemble modelling did not show a significant improvement in the fits.
Storage temperature = UNKNOWN
|
|
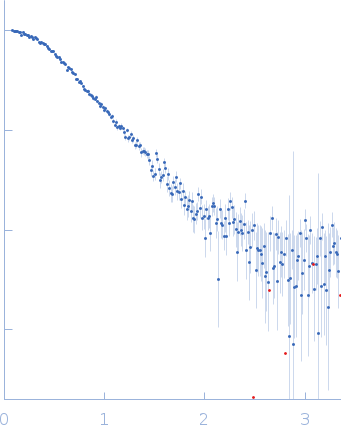 s, nm-1
s, nm-1