|
This dataset is SAXS characterization of the core region (tissue from radial positions of 40%–0% of the total lens radius) of the intact Doryteuthis squid eye lens tissue. The lens contains about 40 unique S-crystallins isoforms that vary in molecular weight due to length variation in a disordered region encoded in the central exon of the coding sequence such that loops form protruding from the folded protein. S-crystallins are dimeric and globular, such that each dimer has two of these disordered loops, which form linking structures between particles in the system. Because the proteins link to form extended materials, even at very low concentration, the parameter Rg does not apply here. Similarly, because this is a measurement of intact biological tissue, there is no experimental buffer per se to subtract, and accordingly, raw data without buffer subtraction are reported here. The pair-distance distribution function (p(r)) was calculated based on unsubtracted data (refer to the full entry zip archive for the p(r) profile). The full set of sequences describing the protein polydispersity in this system is available at GenBank BioProject PRJNA386124. Synchrotron SAXS data were collected on the X9A beam line of the National Synchrotron Light Source (NSLS; Brookhaven, NY, USA) using a 20Hz Pilatus 300K detector at a wavelength of λ = 0.1 nm (I(s) vs s, where s = 4πsinθ/λ and 2θ is the scattering angle). The sample measurement (1 x 300 s exposure) was performed at at 10°C.
The space-filling model ultimately represents the spatial density fluctuation of the material, not the presence of material. Since we measured a nearly water-free density of material at the core of the lens, we show the void density of the model prediction here.
|
|
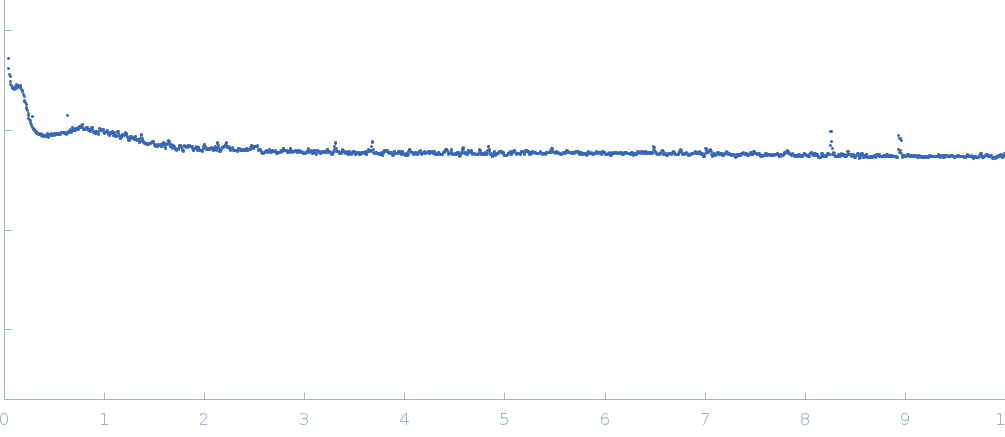 s, nm-1
s, nm-1