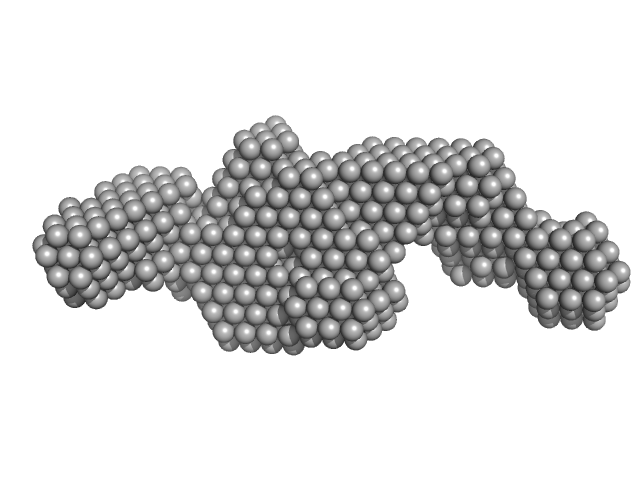
|
Synchrotron SAXS data from solutions of polyglutamine tract-binding protein 1 (PQBP-1) in 20 mM Tris, 150 mM NaCl, 1mM DTT, pH 7 were collected on the EMBL X33 beam line at DORIS III (Hamburg, Germany) using a Pilatus 1M-W detector at a sample-detector distance of 2.7 m and at a wavelength of λ = 0.15 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). Solute concentrations ranging between 7 and 13 mg/ml were measured at 20°C. Eight successive 15 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted. The low angle data collected at lower concentration were merged with the highest concentration high angle data to yield the final composite scattering curve.
|
|
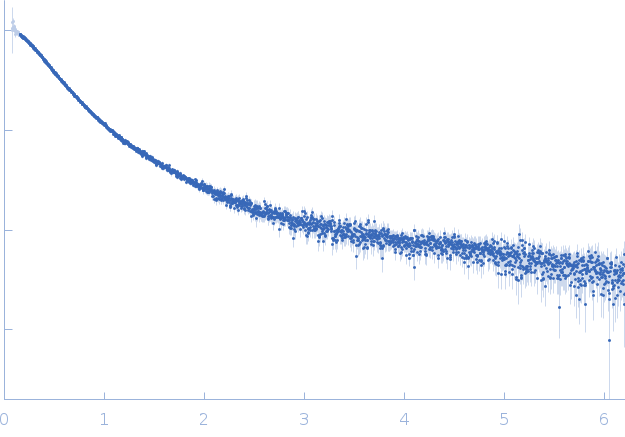 s, nm-1
s, nm-1
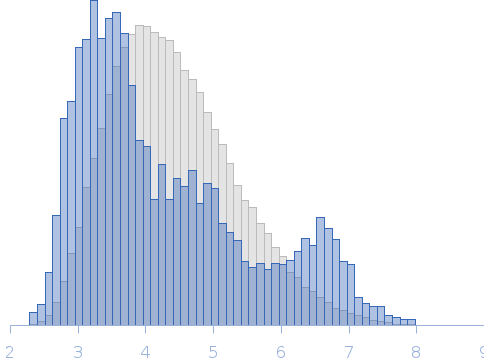 Rg, nm
Rg, nm