|
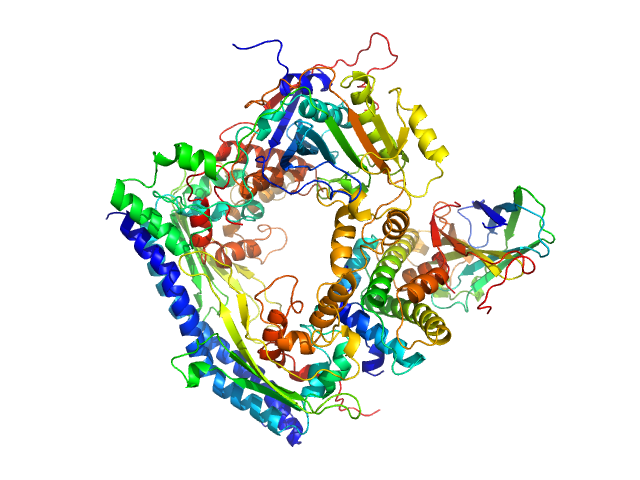
SANS data from solutions the Asf1-H3:H4-Rtt109-Vps75 protein complex (1H Asf1-H3:H4-Rtt109, bound to 70% 2H-labelled Vps75) in 50 mM citrate, 150 mM NaCl, 5 mM BME, 100% D2O, pD 6.5 were collected on the D22 instrument at the ILL (Grenoble, France) using a 3He multidetector 128 linear sensitive Reuter-Stokes detector detector at a sample-detector/Collimation distance of 4 m/4 m and at a wavelength of λ = 0.6 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 4.90 mg/ml was measured at 25°C for 2 h.
|
|
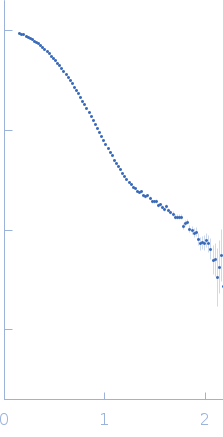 s, nm-1
s, nm-1