|
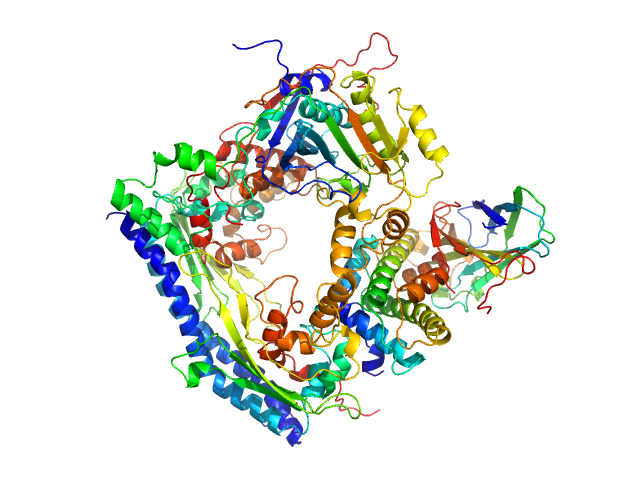
SANS data from solutions of the Asf1-H3:H4-Rtt109-Vps75 protein complex (1H Rtt109-H3:H4, 2H Asf1-Vps75) in 50 mM citrate, 150 mM NaCl, 5 mM BME, 42% D2O, pH 6.5 were collected on the KWS1 instrument the FRM2 (Munich, Germany) using a SANS 6Li-Scintillator 1 mm thickness + photomultiplier detector. Data were collected at two sample detector positions: 1) 1.5 m for 2 hrs using a neutron wavelength of 0.5 nm and; 2) 4 m for 2 hrs using a neutron wavelength of 0.5 nm. Both datasets were recorded on the same sample at 4.7 mg/mL, measured at 25°C.
|
|
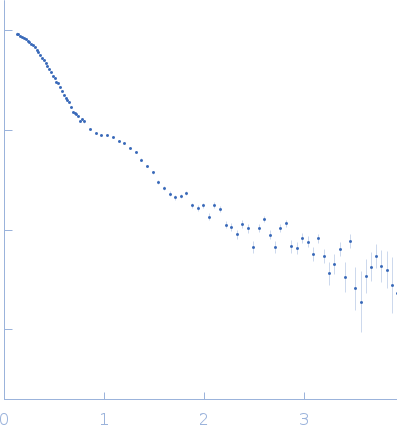 s, nm-1
s, nm-1