| MWI(0) | 160 | kDa |
| MWexpected | 157 | kDa |
| VPorod | 213 | nm3 |
|
log I(s)
1.30×10-1
1.30×10-2
1.30×10-3
1.30×10-4
|
 s, nm-1
s, nm-1
|
|
|
|
|
|
|
|
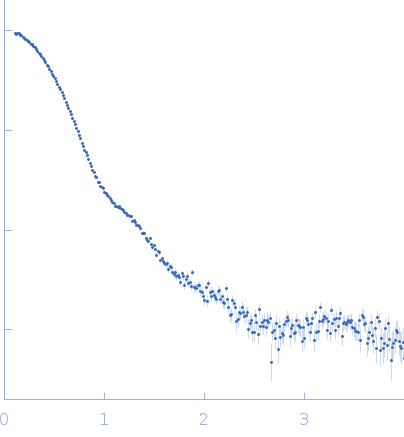
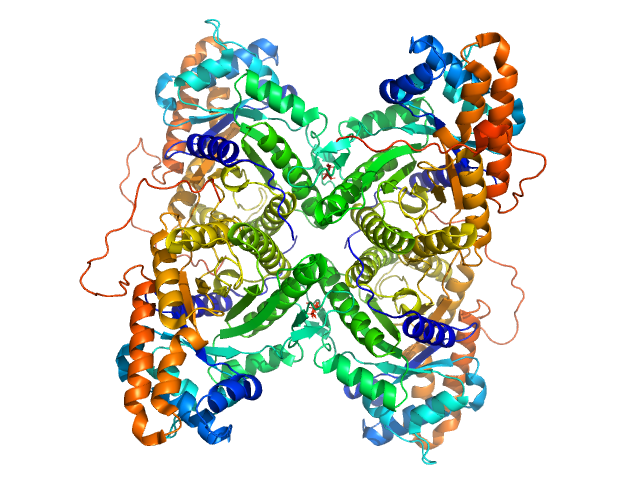
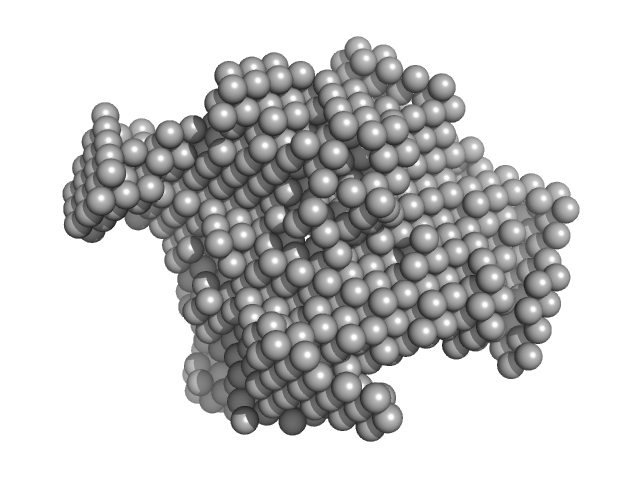
SAXS data from solutions of rabbit muscle fructose-bisphosphate aldolase A (K229M) in 20 mM HEPES, pH 7 were collected using Xenocs BioXolver L instrument with MetalJet (Département de Biochimie, Université de Montréal, Canada) with a Pilatus3 R 300K detector at a wavelength of λ = 1.348 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 8.66 mg/ml was measured at 20°C. 10 successive 60 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
|
|
|||||||||||||||||||||||||||||||||