|
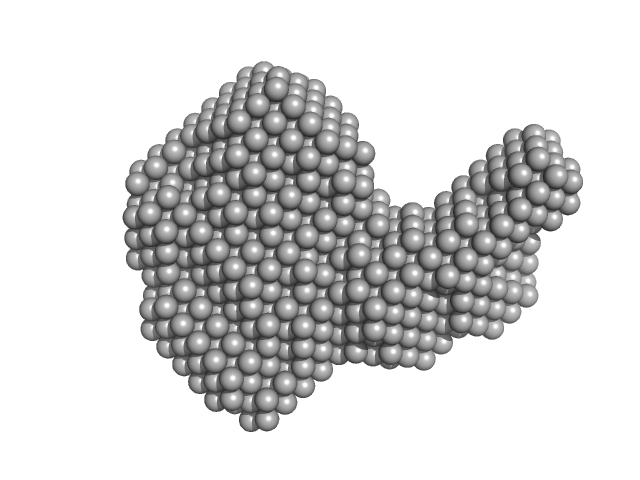
Synchrotron SAXS data from solutions of the Ric-8A (1-491) and G protein complex in 25 mM HEPES, 150 mM NaCl, pH 8 were collected on the BioCAT 18ID beam line at the Advanced Photon Source (APS), Argonne National Laboratory (Lemont, IL, USA) using a Pilatus3 X 1M detector at a sample-detector distance of 3.7 m and at a wavelength of λ = 0.1033 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). In-line size-exclusion chromatography (SEC) SAS was employed. The SEC parameters were as follows: A 500.00 μl sample at 5 mg/ml was injected at a 0.70 ml/min flow rate onto a GE Superdex 200 Increase 10/300 column at 20°C. 1500 successive 0.500 second frames were collected through the elution profile. The data were normalized to the intensity of the transmitted beam and radially averaged and the scattering from an appropriate solvent-blank was subtracted from the 1D scattering intensities obtained for the sample peak.
|
|
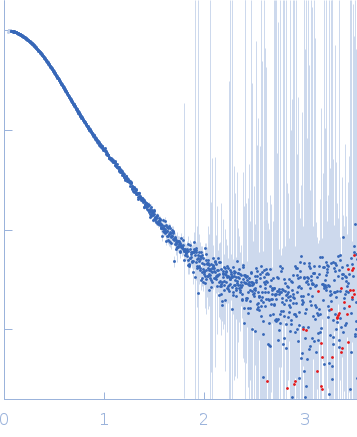 s, nm-1
s, nm-1