|
Synchrotron SAXS data from solutions of the PDZ1-2 domain of postsynaptic density protein 95 (PSD-95) (Paused SEC) in 20 mM TRIS/HCl, 150 mM NaCl, pH 8.5 were collected on the B21 beam line at the Diamond Light Source storage ring (Didcot, UK) using a Pilatus 2M detector at a sample-detector distance of 3.9 m and at a wavelength of λ = 0.1 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). In-line size-exclusion chromatography (SEC) SAS was employed. The SEC parameters were as follows: A 20.00 μl sample at 15 mg/ml was injected at a 0.00 ml/min flow rate onto a Shodex KW403 column . 69 successive 10 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
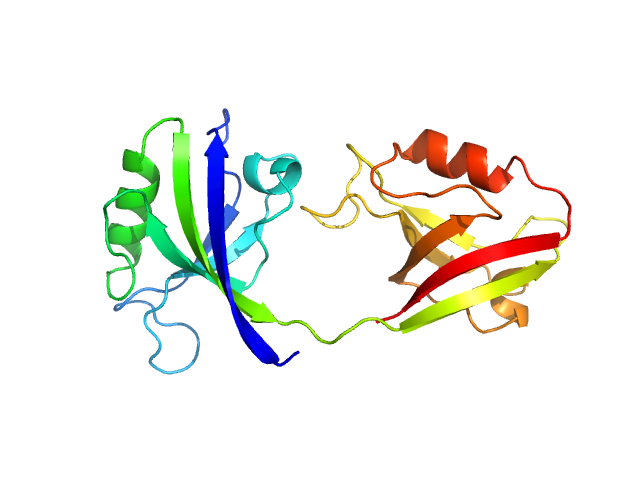
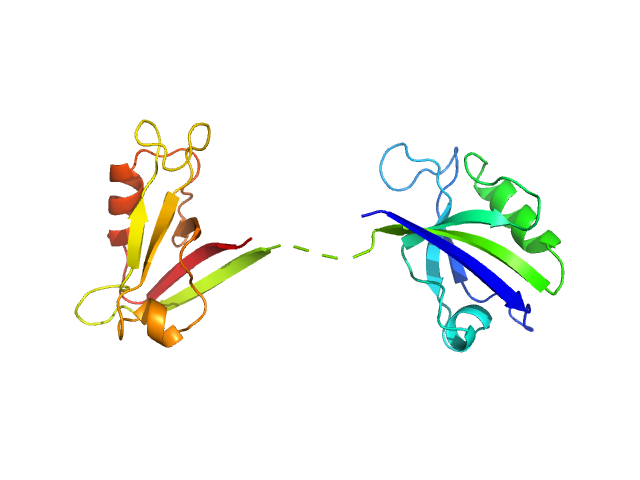
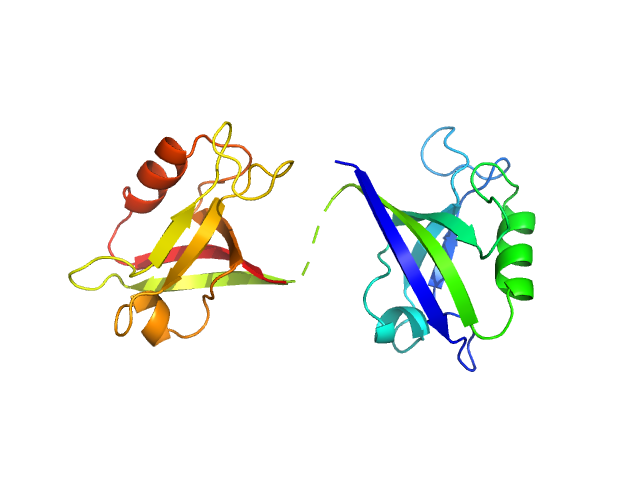
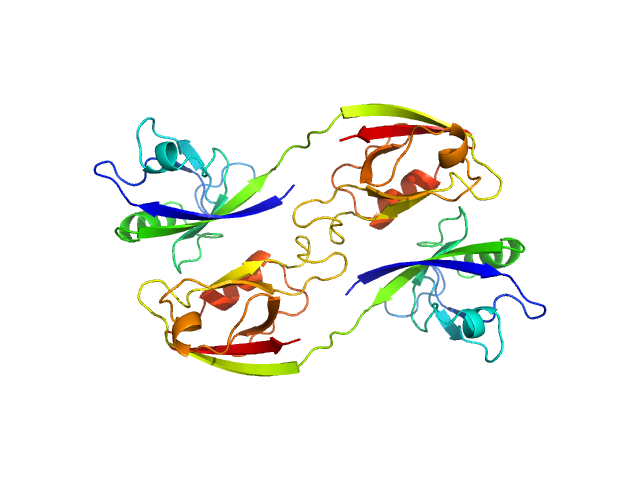
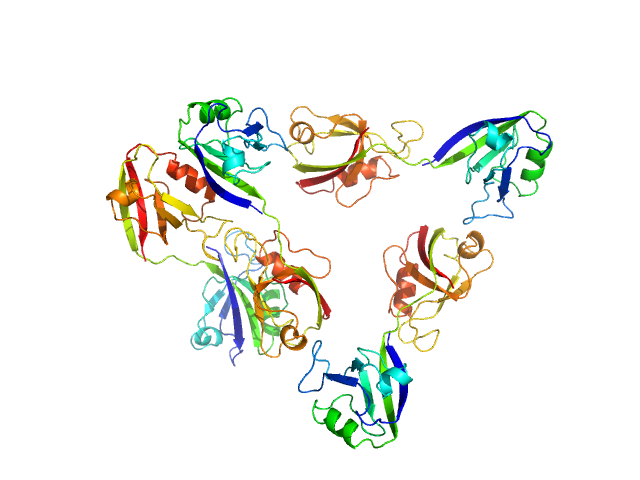
Scattering data are fitted using the ATSAS OLIGOMER program using a suite of models. The ATSAS program FFMAKER was used to generate form factors for each model in the suite. Each model is assigned a volume fraction to fit the observed scattering profile. 3 monomer models are included, the first is a compact conformation of PDZ1-2 similar to PDB entries 6spv/6spz; the other two are domain models obtained from a representative run of the ATSAS EOM program with data projected to infinite dilution. Multimeric models are drawn from a "clustering Spacegroup", unit cell 14.8nm symmetry I2(1)3, and consist of identical copies of an extended conformation of PDZ1-2 (similar to PDB entry 3zrt) assembled by symmetry operations.
In the order of deposition:
Model number; Stoichiometry; MW (kDa); source; Volume fraction.
1; 1; 21; Crystal Structure; 0.382.
2; 1; 21; EOM; 0.106.
3; 1; 21; EOM; 0.397.
4; 2; 42; clustering Spacegroup; 0.081.
5; 4; 84; clustering Spacegroup; 0.034.
|
|
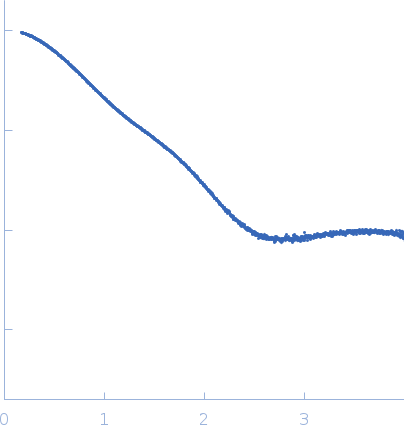 s, nm-1
s, nm-1