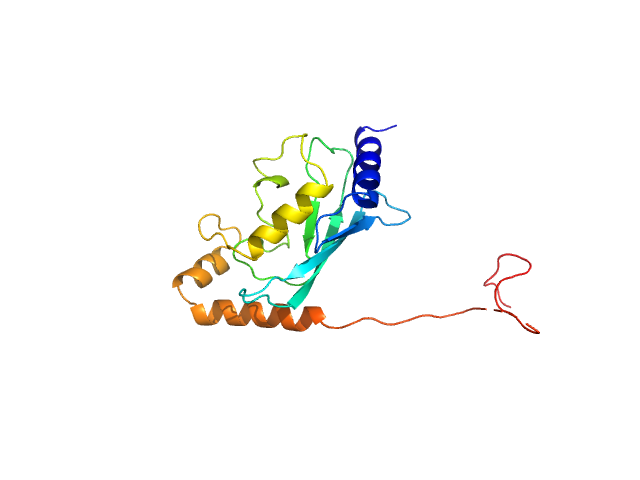
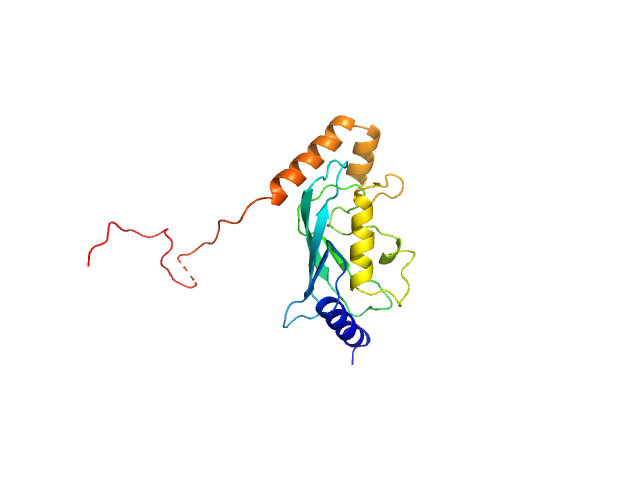
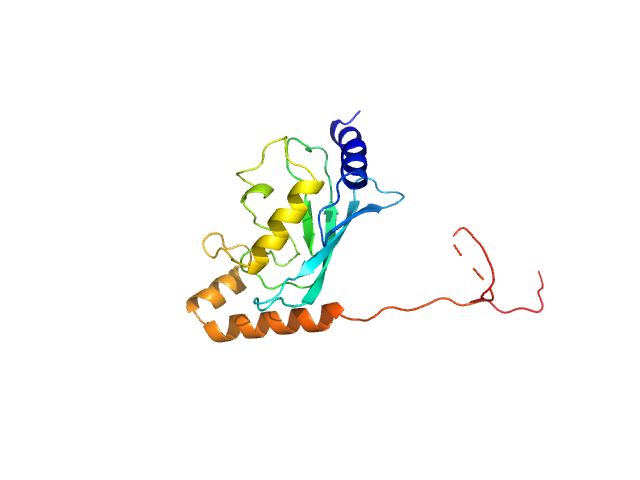
| MWexperimental | 20 | kDa |
| MWexpected | 20 | kDa |
| VPorod | 31 | nm3 |
|
log I(s)
3.09×102
3.09×101
3.09×100
3.09×10-1
|
 s, nm-1
s, nm-1
|
|
|
|
|
|
|
|
|
|
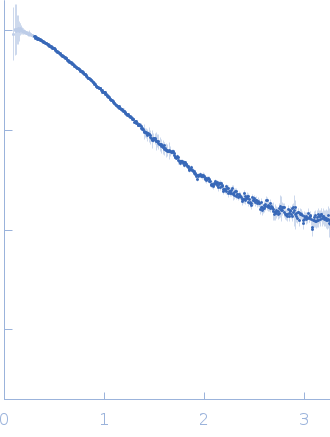
Synchrotron SAXS data from solutions of E2 ubiquitin conjugating enzyme RAD6 in 50 mM Tris, 150 mM NaCl, 1 mM TCEP, pH 7.5 were collected on the 12.3.1 (SIBYLS) beam line at the Advanced Light Source (ALS; Berkeley, CA, USA) using a MAR 165 CCD detector at a sample-detector distance of 1.6 m and at a wavelength of λ = 0.127 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 2.50 mg/ml was measured using exposure times of 1.0, 2.0, 3.0, 4.0 and 5.0 s at 20°C. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering curve of buffer fraction was recorded as reference buffer scattering and was subtracted from sample scattering curve.
Protein was purified on Superdex 200 10/300 column in buffer condition 50 mM Tris pH 7.5 150 mM NaCl, 1mM TCEP. Each fraction was collected and concentrated with centrifugal concentration devices (10 kDa cut-off) to make the protein concentration up to 2.5 mg/ml. Fractions of buffer were also collected on Superdex 200 10/300 column. |
|
|||||||||||||||||||||||||||||||||||||||||||||