|
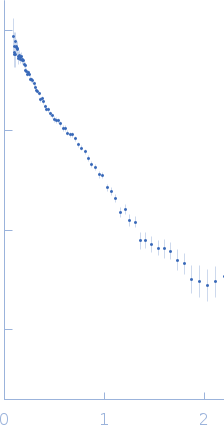
SANS
data from solutions of
Sensory rhodopsin II - transducer complex (NpSRII/NpHtrII) in detergent at 4000 mM NaCl studied with SANS
in
4000 mM NaCl, 100 mM Na/Na-Pi, 1.0 mM EDTA, 0.05% DDM (D2O buffer), pH 8
were collected
using a He3-fulfilled, 8 independent wires detector
at a sample-detector distance of 13.0 m and
at a wavelength of λ = 0.147 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
One solute concentration of 0.40 mg/ml was measured
at 20°C.
Four successive
1800 second frames were collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
The protein of study is a non-fused complex of Sensory rhodopsin II (NpSRII, UniProt ID P42196) with its cognate transducer (NpHtrII, UniProt ID P42259) from Nartonomonas pharaonis.
NpSRII/NpHtrII requires dimerization for signal transduction and forms a trimer of dimers in the N. pharaonis membrane.
The dependence of the oligomeric state of this complex on conditions is the subject of research.
The NpSRII/NpHtrII was co-expressed in E. coli and solubilized in n-Dodecyl β-D-maltoside; therefore, resulting molecular weight may differ from the expected due to the presence of a detergent belt surrounding the tramembrane domain of the complex.
SANS measurements were performed on a YuMO (IBR-2) instrument with a two-detector system (sample-detector distances of 4.5 m and 12.97 m).
The wavelengths used are from 0.5 to 8 Å, the contributions of which are separated using time-of-flight technology.
|
|
 s, nm-1
s, nm-1

