|
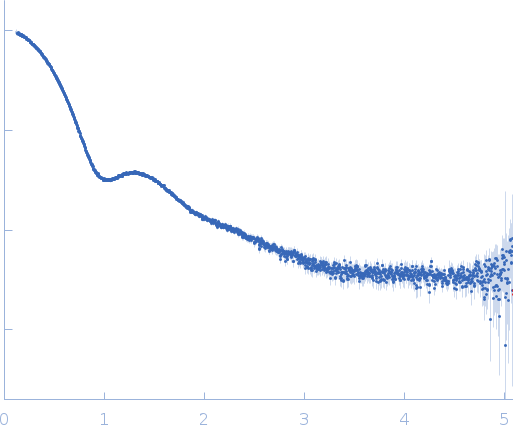
SAXS experiments were carried out at P12 beamline at PETRA-III synchrotron (DESY, Hamburg, Germany). X-ray wavelength was 1.24 Å, the Pilatus 2M detector was positioned 3 m from the sample and the scattering vector ranged from 0.028-7.3 nm-1. Samples (30-40 µL) were measured using robotic sample handler in flow-through mode, to avoid radiation damage. For each sample, data was collected over 40 frames lasting 0.05 s. Frames not displaying any radiation damage were then automatically averaged. Before and after each sample, buffer scattering was collected and subtracted from sample scattering. To assess concentration effects, a dilution series consisting of 4 concentrations was measured. The dilution series were prepared from high concentration (7.5-0.5 mg/mL) stock solutions. No dilution effects were observed.
|
|
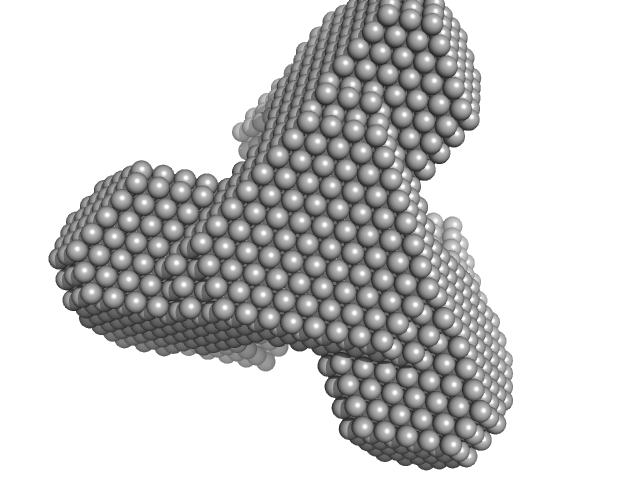
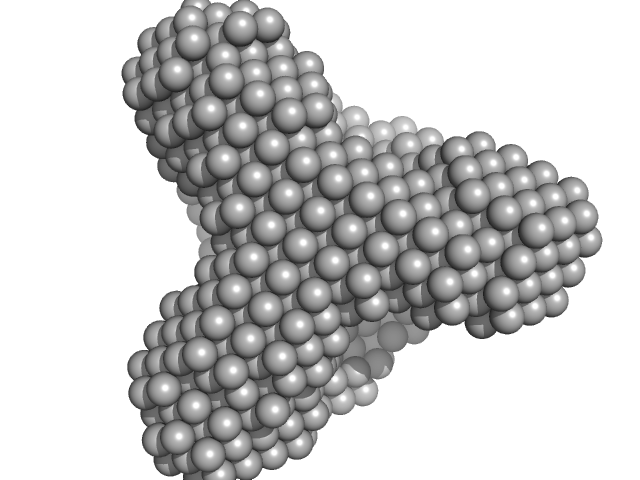
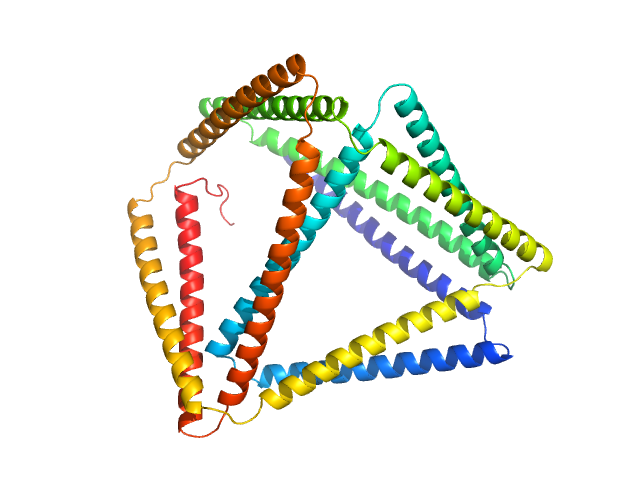
TET12(1.10)SN-f5(22CC)
(TET12SN(22CC))
|
| Mol. type |
|
Protein |
| Organism |
|
synthetic construct |
| Olig. state |
|
Monomer |
| Mon. MW |
|
54.8 kDa |
| Sequence |
|
FASTA |
| |
|
 s, nm-1
s, nm-1