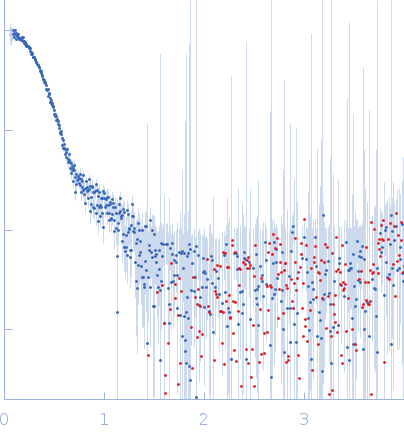
| MWexperimental | 243 | kDa |
| MWexpected | 297 | kDa |
| VPorod | 433 | nm3 |
|
log I(s)
1.12×101
1.12×100
1.12×10-1
1.12×10-2
|
 s, nm-1
s, nm-1
|
|
|
|
|
|
|
|
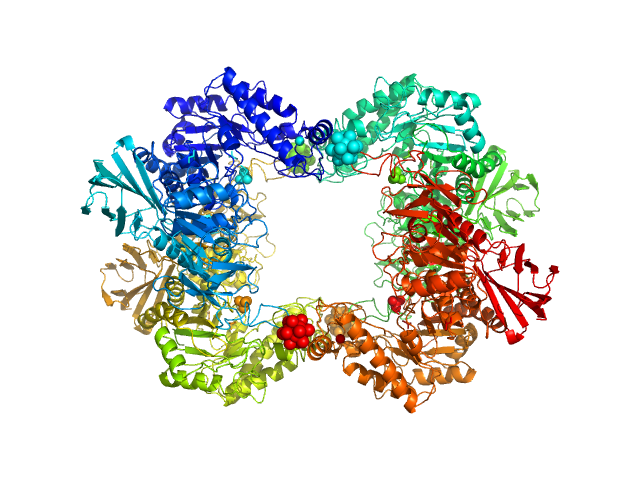
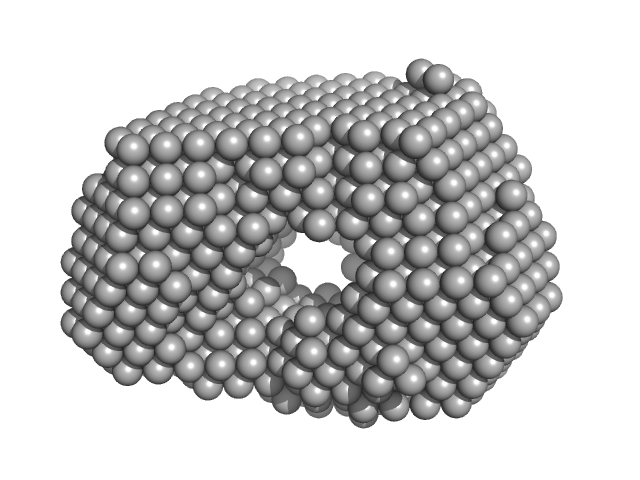
SAXS data from solutions of α-L-Fucosidase isoenzyme 2 (H503A mutant) in 50 mM potassium phosphate, pH 7.4 were collected using an Anton Paar SAXSpoint 2.0 instrument (Institute of Biotechnology, Czech Academy of Sciences, Vestec, Czech Republic) equipped with an Eiger R 1M detector at a sample-detector distance of 0.8 m and at a wavelength of λ = 0.134 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 2.40 mg/ml was measured at 15°C. 15 successive 60 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
Storage temperature = UNKNOWN |
|
|||||||||||||||||||||||||||||||||