|
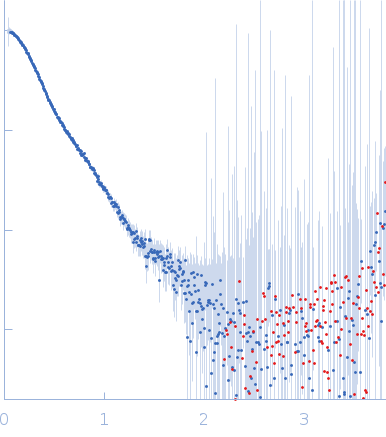
Synchrotron SAXS data from solutions of SFPQ214-598(R542C)/NONO53-312 in 20 mM Tris pH 7.5, 250 mM NaCl, were collected on the SAXS/WAXS beam line at the Australian Synchrotron (Melbourne, Australia) using a Pilatus3 S 2M detector at a sample-detector distance of 3.5 m and at a wavelength of λ = 0.107812 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). In-line size-exclusion chromatography (SEC) SAS was employed. The SEC parameters were as follows: A 60.00 μl sample at 6.8 mg/ml was injected onto a GE Superdex 200 Increase 5/150 column at 25°C. 700 successive 1 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
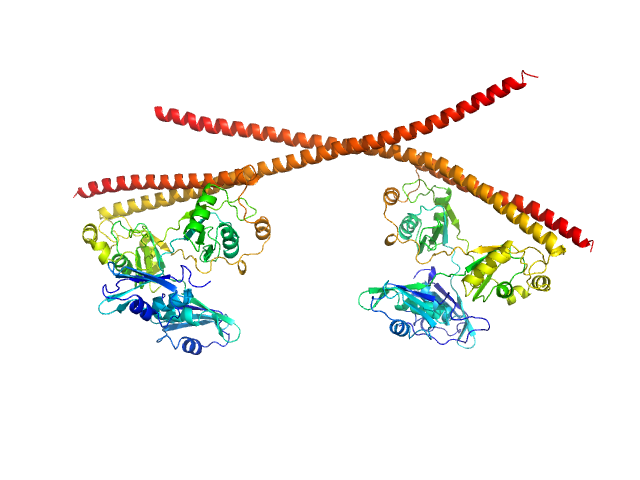
A tetramer of SFPQ214-598(R542C)/NONO53-312 which forms when dimers of SFPQ214-598(R542C)/NONO53-312 bind to each other and become cross-linked by the formation of disulphide bond between the coiled-coil domains of SFPQ.
Imperfect CRYSOL fit of our experimental data to a crystal structure of an SFPQ/NONO tetramer (6WMZ formatted as a tetramer). Our experimental construct contains additional residues (SFPQ214-275) which are not present in the crystal.
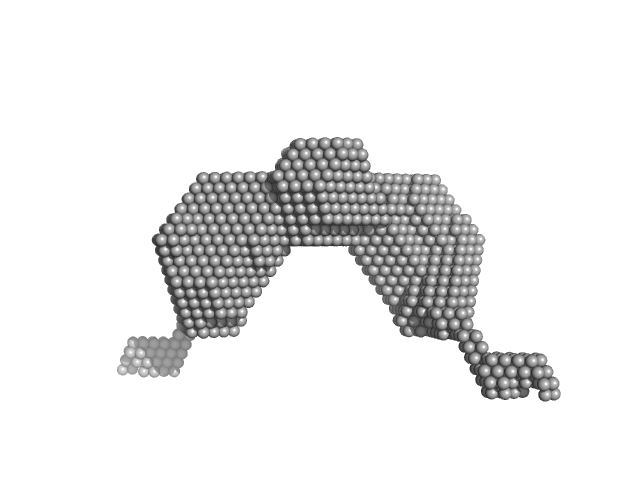
The DAMMIF model conforms to the expected shape of a dumbbell. This matches the general shape of the crystal structure 6WMZ (as a tetramer). The additional regions below the DAMMIF model could be the contributions to the form factor from the DNA-binding domain (SFPQ214-275 which is predicted to be unstructured).
|
|
 s, nm-1
s, nm-1