|
Synchrotron SAXS data from solutions of SFPQ276-565/NONO53-312 in 20 mM Tris pH 7.5, 250 mM NaCl, were collected on the SAXS/WAXS beam line at the Australian Synchrotron (Melbourne, Australia) using a Pilatus3 S 2M detector at a wavelength of λ = 0.10781 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 0.32 mg/ml was measured at 25°C. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
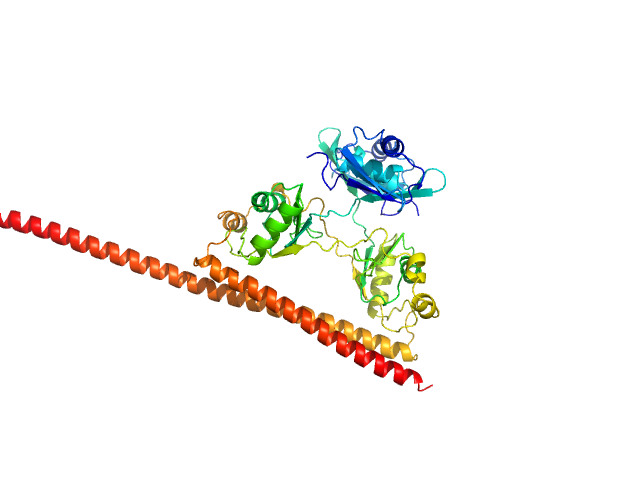
The primary data for this entry shows the SAXS profile of the dimeric form of SFPQ276-565/NONO53-312 that occurs at low protein concentrations (~0.32 mg/ml). At higher sample concentrations, the protein undergoes a concentration dependent self-association into a tetramer as is showcased by a change in scattering and structure. We are aware of this phenomenon because of a cysteine mutated construct (also deposited) which constitutively forms tetramers and previous experiments in the literature. The model we have deposited is a DAMMIF model of the protein at 0.32mg/ml, it matches the approximate dimensions of a truncated dimer of SFPQ276-565/NONO53-312 reasonably well. The full concentration series data ranging up to 6.8mg/ml as well as a P(r) function are made available in the full entry zip archive.
|
|
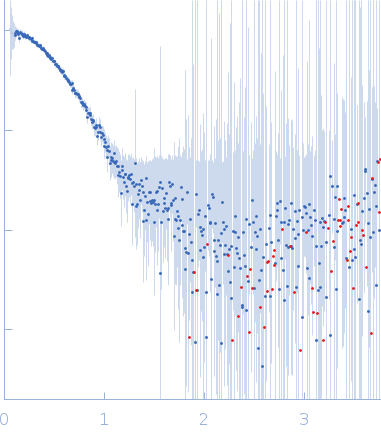 s, nm-1
s, nm-1