|
Synchrotron SAXS data from solutions of SFPQ276-598(R542C)/NONO53-312 at 0.78mg/ml in 20 mM Tris pH 7.5, 250 mM NaCl, were collected on the SAXS/WAXS beam line at the Australian Synchrotron (Melbourne, Australia) using a Pilatus3 S 2M detector at a wavelength of λ = 0.10781 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 0.78 mg/ml was measured at 25°C. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
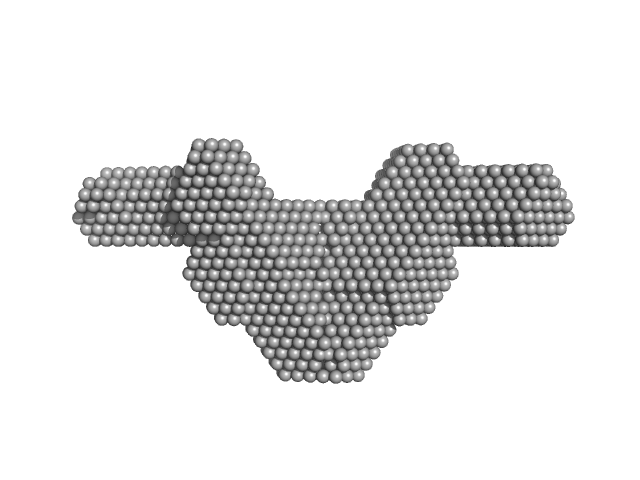
A tetramer of SFPQ276-598(R542C)/NONO53-312 at 0.78mg/ml which forms when dimers of SFPQ276-598(R542C)/NONO53-312 bind to each other and become cross-linked by the formation of disulfide bond between the coiled-coil domains of SFPQ. The P(r) function and other relevant pieces of data are in agreement with those of a similar construct (SFPQ214-598(R542C)/NONO53-312) resolved by SEC-SAXS with a linear Guinier region (deposited). Additionally, the DAMMIF model of our data overlays reasonably well against a crystal structure of the protein formatted as a tetramer (6WMZ).
The rough shape of the molecule appears unchanged as a function of the measured concentration range. We interpret this as the disulfide-linked tetramer persisting over a range of concentrations. Additional data are made available in the full entry zip archive with scattering for the protein at 1.6mg/ml and the corresponding p(r) distribution.
|
|
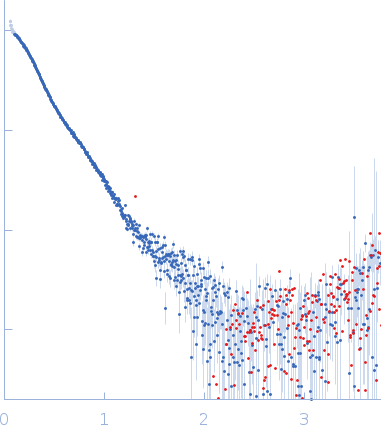 s, nm-1
s, nm-1