|
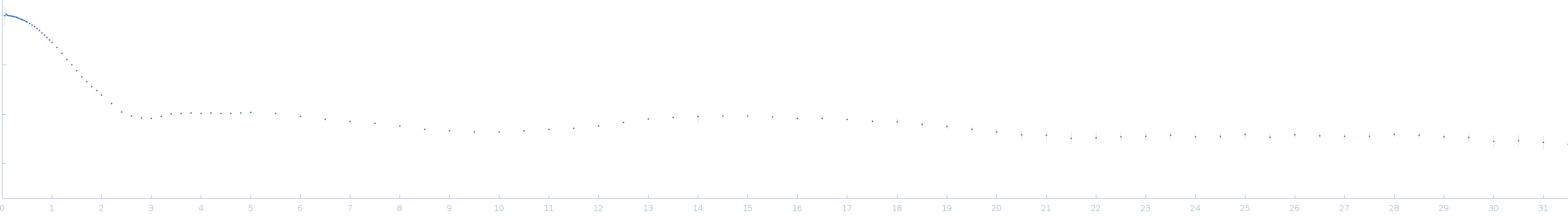
Synchrotron SAXS data from solutions of a ssr1698 Dri1 hemoprotein, wild-type (Zn2+) variant + heme in 50 mM Hepes, 200 mM NaCl, pH 7.5 were collected on the 16-ID (LiX) beam line at the National Synchrotron Light Source II (NSLS-II, Upton, NY, USA) using Pilatus3 X 1M and Pilatus3 X 900K duel detectors at sample-detector distances of 3.760 and 0.343 m, respectively, and at a wavelength of λ = 0.08188 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). In-line size-exclusion chromatography (SEC) SAS was employed. The SEC parameters were as follows: A 90.00 μl sample was injected at a 0.50 ml/min flow rate onto a Biozen 3 µm dSEC-2, 200 Å column at 4°C. Six successive 2 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
|
|
 s, nm-1
s, nm-1