|
Synchrotron SAXS data from solutions of Gramicidin Synthetase 1 (GrsA) W239S mutant in the apo form in 20 mM Tris pH 7.5, 100 mM NaCl, 10 mM MgCl2, 2% glycerol, were collected on the 12.3.1 (SIBYLS) beam line at the Advanced Light Source (ALS; Berkeley, CA, USA) using a Pilatus3 X 2M detector at a sample-detector distance of 2.1 m and at a wavelength of λ = 0.1127 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 5.00 mg/ml was measured at 7.9°C. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
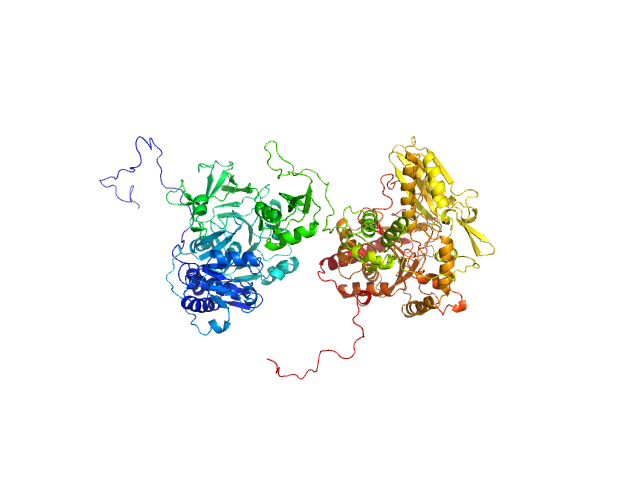
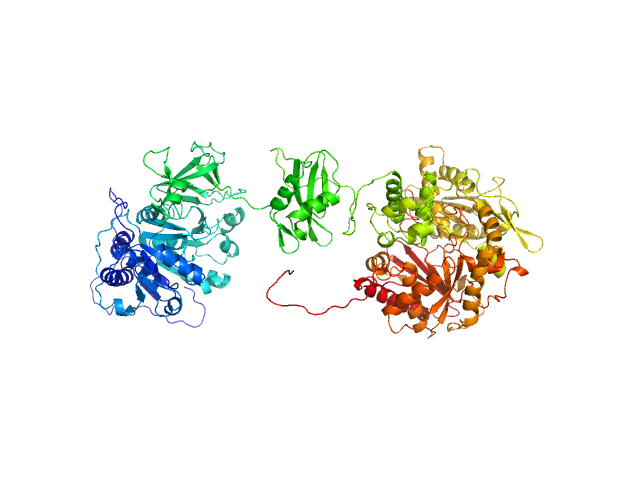
The apo form of GrsA-W239S exists in an ensemble of 2 states as determined by bilboMD. Individually, the compact form is present as 51% of the representative species with Dmax of 14.6 nmand Rg of 3.93 nm, while the elongated form was present as 49% of the ensemble with Dmax of 15.4 nm and Rg of 4.69 nm. The atomic models displayed in this entry represent the compact form (top) and elongated form (bottom).
|
|
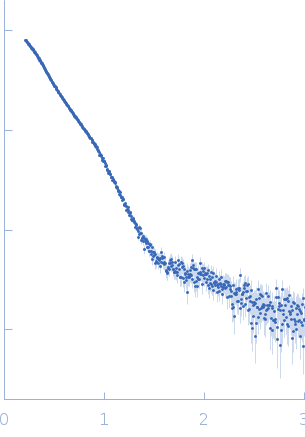 s, nm-1
s, nm-1