|
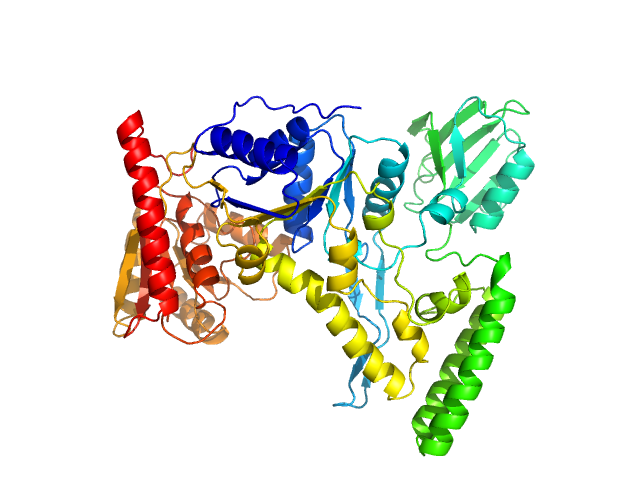
SAXS data from solutions of UvrB (UvrABC system protein B) in 50 mM Tris pH-8, 200 mM NaCl, 2% glycerol were collected using an Anton Paar SAXSpace instrument at the CSIR-Central Drug Research Institute (Lucknow, India) equipped with a Mythen2 R 1K detector at a sample-detector distance of 0.3 m and at a wavelength of λ = 0.154 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 5.00 mg/ml was measured at 16°C. Two successive 1800 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
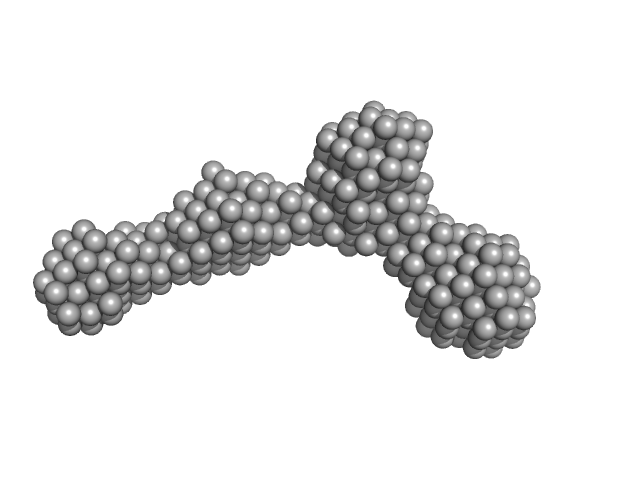
CAUTION: Dmax severely underestimated. CAUTION: The DAM model displayed in this entry did not generate the corresponding fit to the SAXS data, but represents the volume and bead-occupancy corrected shape derived from the spatial alignment of an unknown number of individual models.
|
|
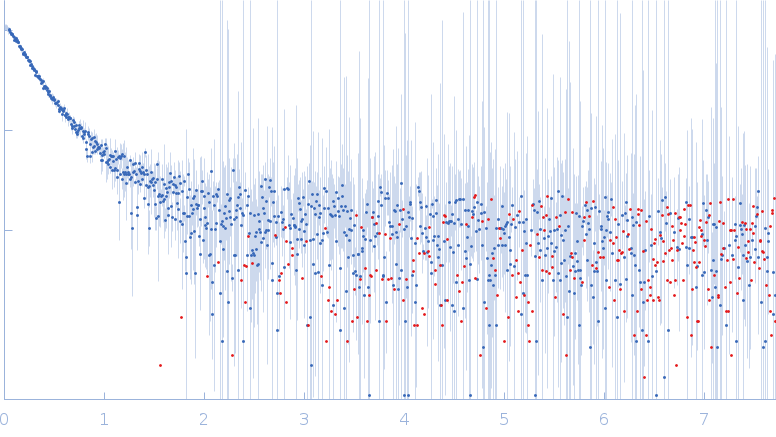 s, nm-1
s, nm-1