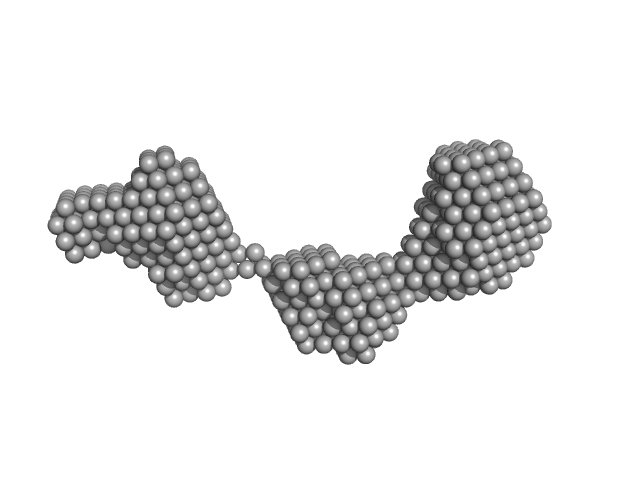
|
Synchrotron SAXS data from solutions of C-terminally truncated Apolipoprotein A-I in 20 mM Tris, 150 mM NaCl, 0.1% sodium azide, pH 7.4 were collected on the SAXS/WAXS beam line at the Australian Synchrotron (Melbourne, Australia) using a Pilatus3 S 2M detector at a wavelength of λ = 0.1078 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). In-line size-exclusion chromatography (SEC) SAS was employed. The SEC parameters were as follows: A 50.00 μl sample at 5 mg/ml was injected at a 0.40 ml/min flow rate onto a GE Superdex 200 Increase 5/150 column at 20°C. 12 successive 1 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
Storage temperature = UNKNOWN. Sample detector distance = UNKNOWN
|
|
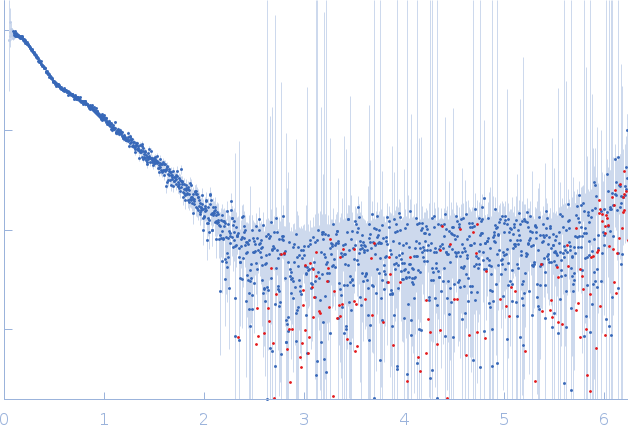 s, nm-1
s, nm-1