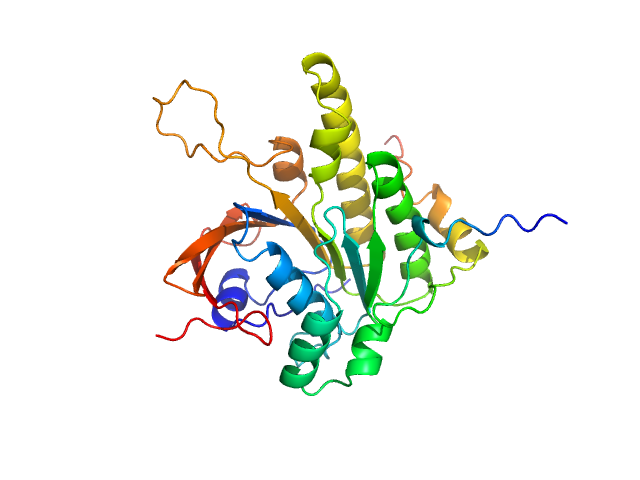
|
Synchrotron SAXS
data from solutions of
N-terminal truncated RAD51 in complex with fourth BRC repeat (BRC4) SEC-SAXS
in
20 mM K₂HPO₄/KH₂PO₄, 100 mM NaCl, 200 mM Li₂SO₄, 1 mM DTT, 2% sucrose, pH 8
were collected
on the
B21 beam line
at the Diamond Light Source storage ring
(Didcot, UK)
using a Eiger 4M detector
at a sample-detector distance of 36.9 m and
at a wavelength of λ = 0.09464 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
In-line size-exclusion chromatography (SEC) SAS was employed. The SEC parameters were as follows: A 100.00 μl sample
at 6.6 mg/ml was injected at a 0.07 ml/min flow rate
onto a Cytiva Superdex 200 Increase 3.2/300 column
at 15°C.
600 successive
3 second frames were collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
Molecular weight has been inferred through Volume of Correlation (Vc). Sample molecular weight and monodispersity has been independently confirmed through static light scattering (SLS). 52 successive frames were collected through the sample elution peak (from a total of 600 frames).
|
|
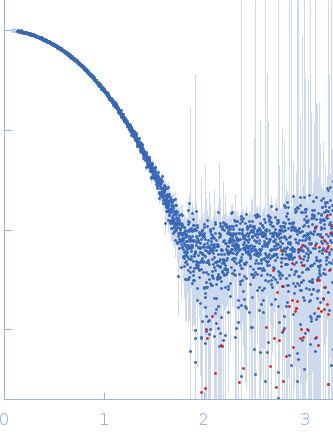 s, nm-1
s, nm-1