| MWexperimental | 10 | kDa |
| MWexpected | 7 | kDa |
| VPorod | 11 | nm3 |
|
log I(s)
1.16×10-2
1.16×10-3
1.16×10-4
1.16×10-5
|
 s, nm-1
s, nm-1
|
|
|
|
|
|

|
|

|
|
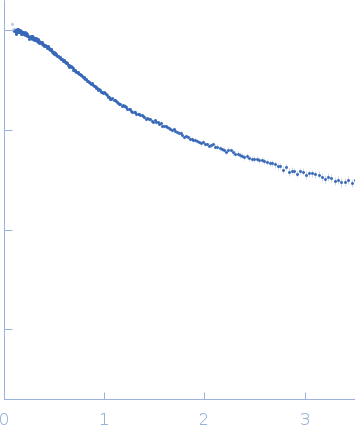
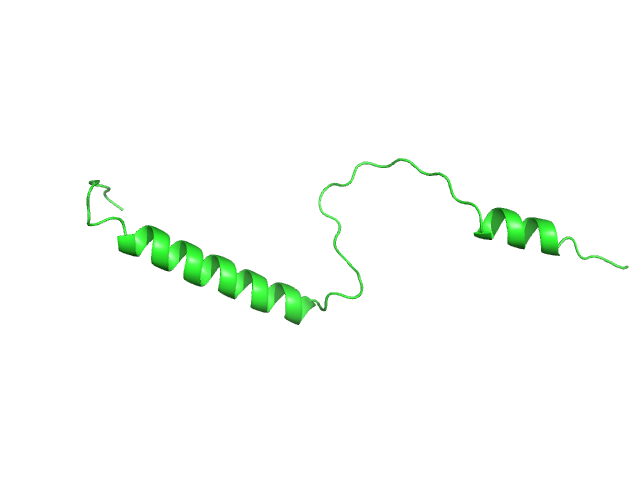
Synchrotron SAXS data from solutions of SERF1a 20 mM sodium phosphate, 20 mM NaCl, pH 6.8 were collected on the 13A beam line at the Taiwan Photon Source, NSRRC (Hsinchu, Taiwan) using a Eiger X 9M detector at a sample-detector distance of 2.5 m and at a wavelength of λ = 0.08266 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 15.00 mg/ml was measured at 10°C. Five successive 2 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
|
|
|||||||||||||||||||||||||||