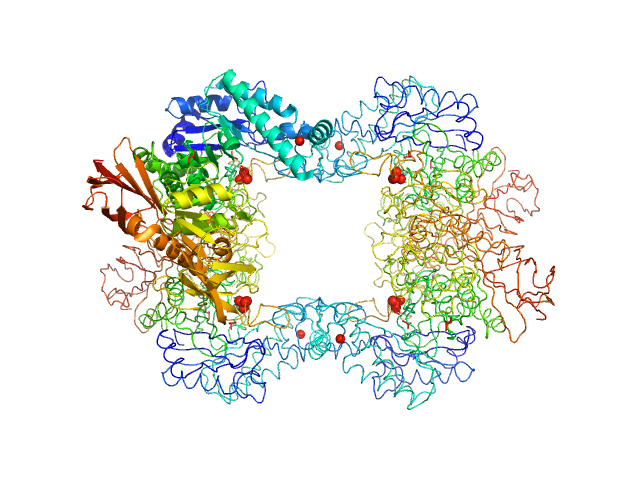
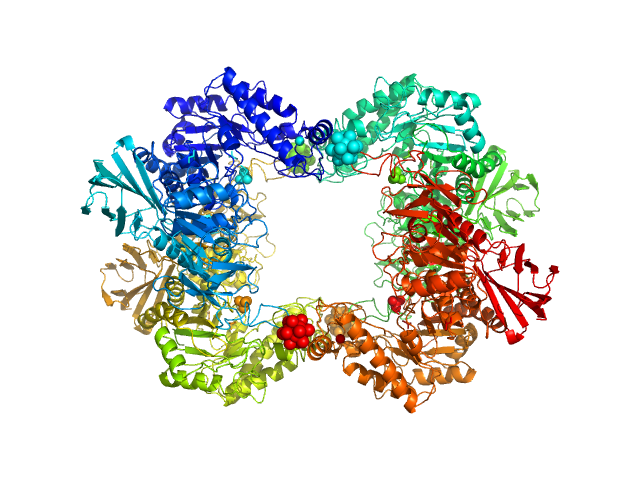
The first structure–function study of GH151 α‐ l
‐fucosidase uncovers new oligomerization pattern, active site complementation, and selective substrate specificity
Koval'ová T,
Kovaľ T,
Stránský J,
Kolenko P,
Dušková J,
Švecová L,
Vodičková P,
Spiwok V,
Benešová E,
Lipovová P,
Dohnálek J
The FEBS Journal
(2022 Feb 20)
|
|
|
|
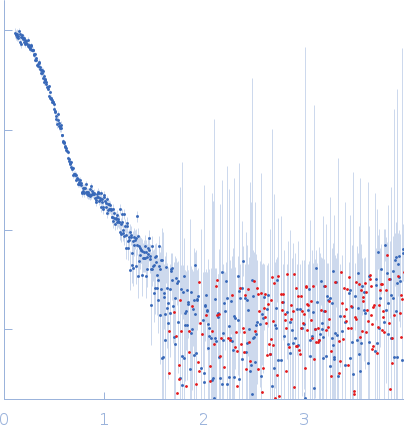
|
| Sample: |
Alpha-L-fucosidase tetramer, 297 kDa Paenibacillus thiaminolyticus (Bacillus … protein
|
| Buffer: |
50 mM potassium phosphate, pH: 7.4 |
| Experiment: |
SAXS
data collected at Anton Paar SAXSpoint 2.0, Institute of Biotechnology, Czech Academy of Sciences/Centre of Molecular Structure on 2019 Jul 16
|
|
| RgGuinier |
4.2 |
nm |
| Dmax |
13.2 |
nm |
| VolumePorod |
418 |
nm3 |
|
|
|
|
|
|
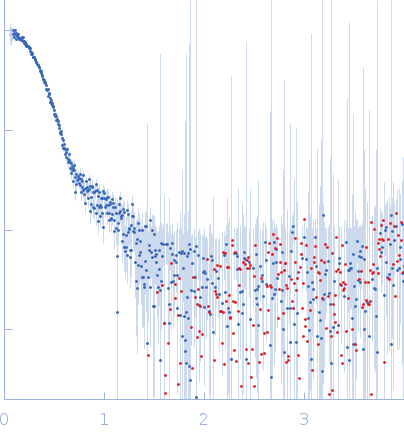
|
| Sample: |
Alpha-L-fucosidase H503A tetramer, 297 kDa Paenibacillus thiaminolyticus (Bacillus … protein
|
| Buffer: |
50 mM potassium phosphate, pH: 7.4 |
| Experiment: |
SAXS
data collected at Anton Paar SAXSpoint 2.0, Institute of Biotechnology, Czech Academy of Sciences/Centre of Molecular Structure on 2019 Jul 16
|
|
| RgGuinier |
4.5 |
nm |
| Dmax |
13.5 |
nm |
| VolumePorod |
433 |
nm3 |
|
|