Almost half of the RTX domain is dispensable for complement receptor 3 binding and cell-invasive activity of the adenylate cyclase toxin.
Espinosa-Vinals CA,
Masin J,
Holubova J,
Stanek O,
Jurnecka D,
Osicka R,
Sebo P,
Bumba L
J Biol Chem
:100833
(2021 May 26)
|
|
|
|
|
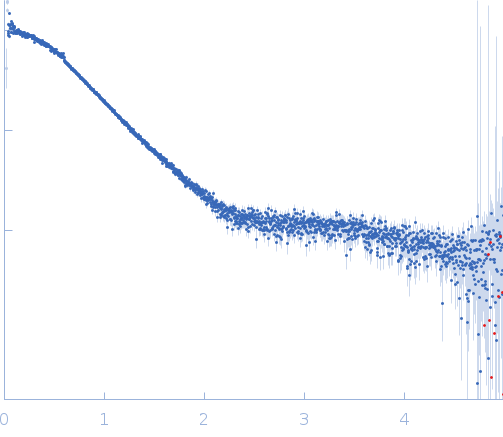
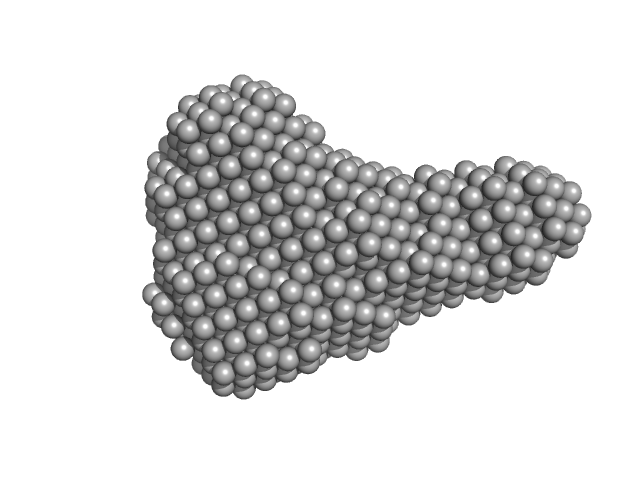
| Sample: |
Hybrid RTX-1 construct (amino acids 1132-1294 and 1562-1681 of CyaA) monomer, 30 kDa Bordetella pertussis protein
|
| Buffer: |
10 mM Tris HCl, 150 mM NaCl, 10 mM CaCl₂, pH: 8 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2016 Dec 1
|
|
| RgGuinier |
2.4 |
nm |
| Dmax |
8.2 |
nm |
| VolumePorod |
41 |
nm3 |
|
|
|
|
|
|
|
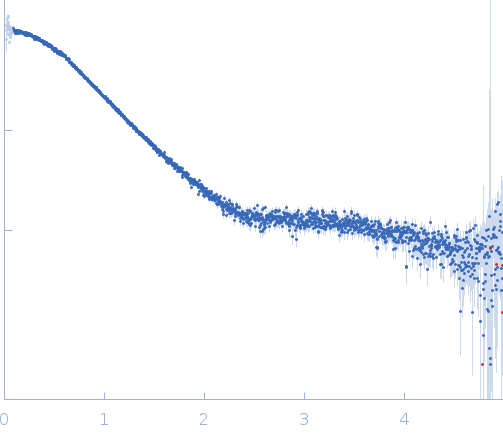
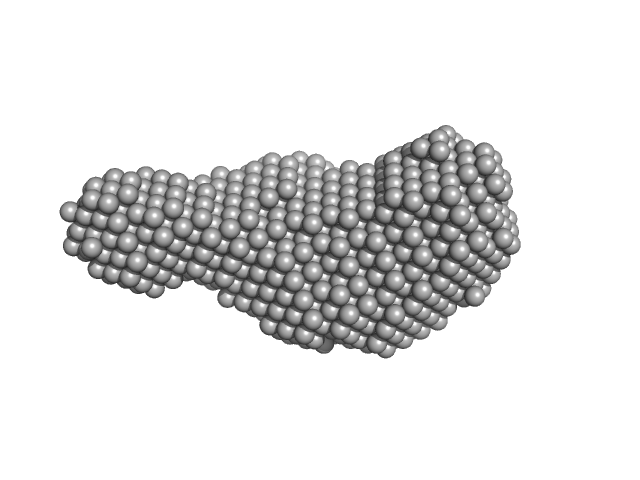
| Sample: |
Hybrid RTX-2 construct (amino acids 1132-1303 and 1562-1681 of CyaA) monomer, 31 kDa Bordetella pertussis protein
|
| Buffer: |
10 mM Tris HCl, 150 mM NaCl, 10 mM CaCl₂, pH: 8 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2016 Dec 1
|
|
| RgGuinier |
2.3 |
nm |
| Dmax |
8.0 |
nm |
| VolumePorod |
37 |
nm3 |
|
|