Structural complexity of the co-chaperone SGTA: a conserved C-terminal region is implicated in dimerization and substrate quality control.
Martínez-Lumbreras S,
Krysztofinska EM,
Thapaliya A,
Spilotros A,
Matak-Vinkovic D,
Salvadori E,
Roboti P,
Nyathi Y,
Muench JH,
Roessler MM,
Svergun DI,
High S,
Isaacson RL
BMC Biol
16(1):76
(2018 Jul 11)
|
|
|
|
|
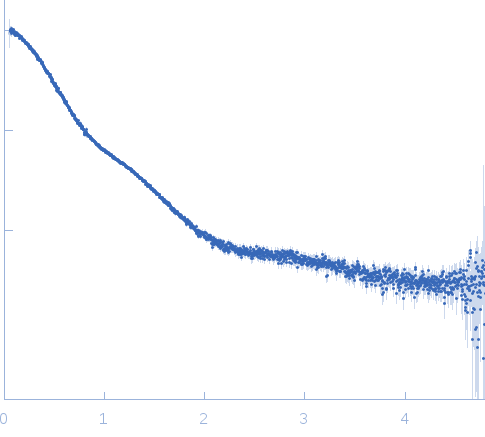
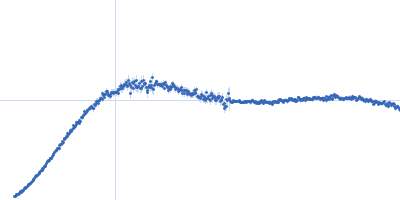
| Sample: |
Small glutamine-rich tetratricopeptide repeat-containing protein alpha full length dimer, 68 kDa Homo sapiens protein
|
| Buffer: |
10 mM potassium phosphate, 100 mM NaCl, pH: 6 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2015 Jun 5
|
|
|
|
|
|
|
|
|
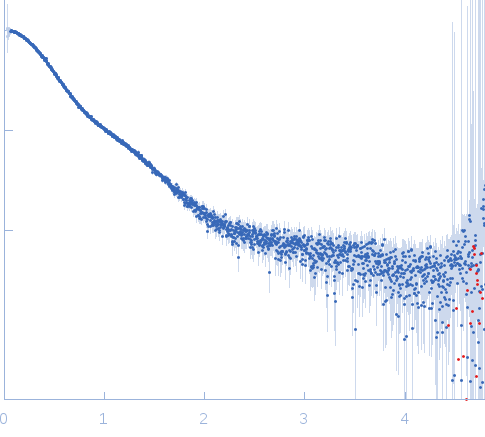
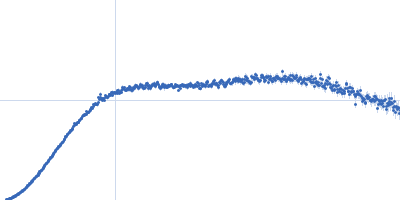
| Sample: |
Small glutamine-rich tetratricopeptide repeat-containing protein alpha Nterminal-TPR domains dimer, 47 kDa Homo sapiens protein
|
| Buffer: |
10 mM potassium phosphate, 100 mM NaCl, pH: 6 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2015 Jun 5
|
|
|
|