|
Synchrotron SAXS
data from solutions of
Dihydroneopterin aldolase from H.pylori (HpDHNA:Pterin)
in
25 mM Tris-HCl pH 7.5 and 150 mM NaCl, pH 7.5
were collected
on the
12-ID-B SAXS/WAXS beam line
at the Advanced Photon Source (APS), Argonne National Laboratory storage ring
(Lemont, IL, USA)
using a Pilatus 2M detector
at a sample-detector distance of 2 m and
at a wavelength of λ = 0.0932 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
Solute concentrations ranging between 0.5 and 4 mg/ml were measured
at 20°C.
45 successive
1 second frames were collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
The low angle data collected at lower concentrations were extrapolated to infinite dilution and merged with the higher concentration data to yield the final composite scattering curve.
Molecular weight based on Vc method is 55.3KDa
Molecular weight based on apparent volume method is 53.4KDa
|
|
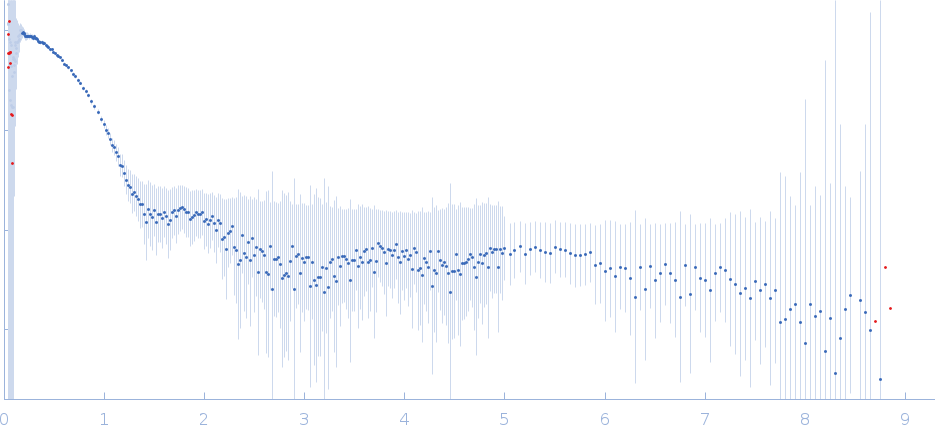 s, nm-1
s, nm-1