UniProt ID: None (2-136) Histone H3
UniProt ID: P62799 (2-103) Histone H4
UniProt ID: Q6AZJ8 (2-130) Histone H2a
UniProt ID: Q92130 (5-126) Histone H2b
UniProt ID: None (None-None) 147bp 601 Widom sequence
|
|
|
|
| Sample: |
Histone H3 dimer, 31 kDa Xenopus laevis protein
Histone H4 dimer, 22 kDa Xenopus laevis protein
Histone H2a dimer, 28 kDa Xenopus laevis protein
Histone H2b dimer, 27 kDa Xenopus laevis protein
147bp 601 Widom sequence monomer, 45 kDa synthetic construct DNA
|
| Buffer: |
15 mM HEPES, 200 mM NaCl, pH: 7.3 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Dec 11
|
A Tail-Based Mechanism Drives Nucleosome Demethylation by the LSD2/NPAC Multimeric Complex.
Cell Rep 27(2):387-399.e7 (2019)
Marabelli C, Marrocco B, Pilotto S, Chittori S, Picaud S, Marchese S, Ciossani G, Forneris F, Filippakopoulos P, Schoehn G, Rhodes D, Subramaniam S, Mattevi A
|
| RgGuinier |
4.4 |
nm |
| Dmax |
12.8 |
nm |
| VolumePorod |
353 |
nm3 |
|
|
UniProt ID: Q8NB78 (31-822) Lysyne-specific Demethylase LSD2
UniProt ID: Q49A26 (205-553) NPAC linker+DH (delta-205)
UniProt ID: None (2-136) Histone H3
UniProt ID: P62799 (2-103) Histone H4
UniProt ID: Q6AZJ8 (2-130) Histone H2a
UniProt ID: Q92130 (5-126) Histone H2b
UniProt ID: None (None-None) 147bp 601 Widom sequence
|
|
|
|
| Sample: |
Lysyne-specific Demethylase LSD2 monomer, 89 kDa Homo sapiens protein
NPAC linker+DH (delta-205) tetramer, 150 kDa Homo sapiens protein
Histone H3 dimer, 31 kDa Xenopus laevis protein
Histone H4 dimer, 22 kDa Xenopus laevis protein
Histone H2a dimer, 28 kDa Xenopus laevis protein
Histone H2b dimer, 27 kDa Xenopus laevis protein
147bp 601 Widom sequence monomer, 45 kDa synthetic construct DNA
|
| Buffer: |
15 mM HEPES, 200 mM NaCl, pH: 7.3 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Dec 11
|
A Tail-Based Mechanism Drives Nucleosome Demethylation by the LSD2/NPAC Multimeric Complex.
Cell Rep 27(2):387-399.e7 (2019)
Marabelli C, Marrocco B, Pilotto S, Chittori S, Picaud S, Marchese S, Ciossani G, Forneris F, Filippakopoulos P, Schoehn G, Rhodes D, Subramaniam S, Mattevi A
|
| RgGuinier |
7.8 |
nm |
| Dmax |
30.4 |
nm |
| VolumePorod |
1100 |
nm3 |
|
|
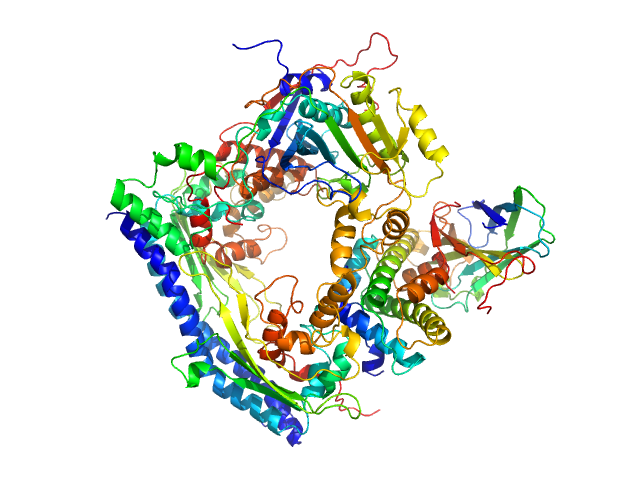
UniProt ID: P53853 (1-225) Vacuolar protein sorting-associated protein 75 (1-225 aa)
UniProt ID: Q07794 (1-436) Histone acetyltransferase RTT109
UniProt ID: P32447 (1-169) Histone chaperone ASF1
UniProt ID: P84233 (35-135) Histone H3.2 (35-135 aa)
UniProt ID: P62799 (1-103) Histone H4
|
|
|
|
| Sample: |
Vacuolar protein sorting-associated protein 75 (1-225 aa) dimer, 53 kDa Saccharomyces cerevisiae protein
Histone acetyltransferase RTT109 monomer, 50 kDa Saccharomyces cerevisiae protein
Histone chaperone ASF1 monomer, 19 kDa protein
Histone H3.2 (35-135 aa) monomer, 12 kDa Xenopus laevis protein
Histone H4 monomer, 11 kDa Xenopus laevis protein
|
| Buffer: |
50 mM citrate, 150 mM NaCl, 5 mM BME, 100% D2O, pH: 6.5 |
| Experiment: |
SANS
data collected at D22, Institut Laue-Langevin (ILL) on 2016 Nov 14
|
Histone chaperone exploits intrinsic disorder to switch acetylation specificity (Asf1-H3:H4-Rtt109-Vps75 protein complex, data for docking block selections)
Nat Commun 10(1):3435 (2019)
Danilenko N, Lercher L, Kirkpatrick J, Gabel F, Codutti L, Carlomagno T
|
| RgGuinier |
1.9 |
nm |
| Dmax |
5.5 |
nm |
|
|
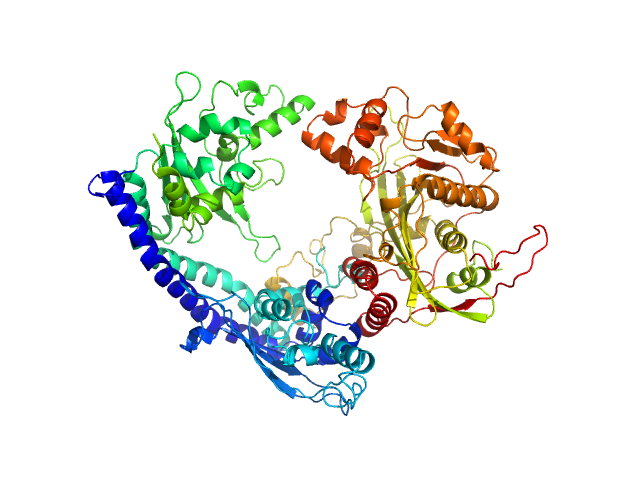
UniProt ID: P53853 (1-225) Vacuolar protein sorting-associated protein 75 (1-225 aa)
UniProt ID: Q07794 (1-436) Histone acetyltransferase RTT109
UniProt ID: P32447 (1-169) Histone chaperone ASF1
UniProt ID: P62799 (1-103) Histone H4
UniProt ID: Q6PI79 (1-136) Histone H3 full-length
|
|
|
|
| Sample: |
Vacuolar protein sorting-associated protein 75 (1-225 aa) dimer, 53 kDa Saccharomyces cerevisiae protein
Histone acetyltransferase RTT109 monomer, 50 kDa Saccharomyces cerevisiae protein
Histone chaperone ASF1 monomer, 19 kDa protein
Histone H4 monomer, 11 kDa Xenopus laevis protein
Histone H3 full-length monomer, 15 kDa Xenopus laevis protein
|
| Buffer: |
50 mM citrate, 150 mM NaCl, 5 mM BME, 42% D2O, pH: 6.5 |
| Experiment: |
SANS
data collected at D22, Institut Laue-Langevin (ILL) on 2016 Nov 14
|
Histone chaperone exploits intrinsic disorder to switch acetylation specificity (Asf1-H3:H4-Rtt109-Vps75 protein complex, data for docking block selections)
Nat Commun 10(1):3435 (2019)
Danilenko N, Lercher L, Kirkpatrick J, Gabel F, Codutti L, Carlomagno T
|
| RgGuinier |
3.4 |
nm |
| Dmax |
10.5 |
nm |
|
|
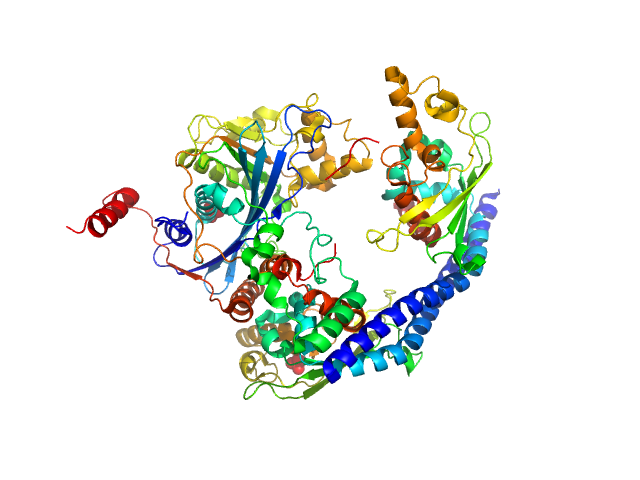
UniProt ID: P53853 (1-225) Vacuolar protein sorting-associated protein 75 (1-225 aa)
UniProt ID: Q07794 (1-436) Histone acetyltransferase RTT109
UniProt ID: P32447 (1-169) Histone chaperone ASF1
UniProt ID: P62799 (1-103) Histone H4
UniProt ID: Q6PI79 (1-136) Histone H3 full-length
|
|
|
|
| Sample: |
Vacuolar protein sorting-associated protein 75 (1-225 aa) dimer, 53 kDa Saccharomyces cerevisiae protein
Histone acetyltransferase RTT109 monomer, 50 kDa Saccharomyces cerevisiae protein
Histone chaperone ASF1 monomer, 19 kDa protein
Histone H4 monomer, 11 kDa Xenopus laevis protein
Histone H3 full-length monomer, 15 kDa Xenopus laevis protein
|
| Buffer: |
50 mM citrate, 150 mM NaCl, 5 mM BME, 42% D2O, pH: 6.5 |
| Experiment: |
SANS
data collected at D22, Institut Laue-Langevin (ILL) on 2016 Nov 14
|
Histone chaperone exploits intrinsic disorder to switch acetylation specificity (Asf1-H3:H4-Rtt109-Vps75 protein complex, data for docking block selections)
Nat Commun 10(1):3435 (2019)
Danilenko N, Lercher L, Kirkpatrick J, Gabel F, Codutti L, Carlomagno T
|
| RgGuinier |
2.7 |
nm |
| Dmax |
9.0 |
nm |
|
|
UniProt ID: P53853 (1-225) Vacuolar protein sorting-associated protein 75 (1-225 aa)
UniProt ID: Q07794 (1-436) Histone acetyltransferase RTT109
UniProt ID: P32447 (1-169) Histone chaperone ASF1
UniProt ID: P62799 (1-103) Histone H4
UniProt ID: Q6PI79 (1-136) Histone H3 full-length
|
|
|
|
| Sample: |
Vacuolar protein sorting-associated protein 75 (1-225 aa) dimer, 53 kDa Saccharomyces cerevisiae protein
Histone acetyltransferase RTT109 monomer, 50 kDa Saccharomyces cerevisiae protein
Histone chaperone ASF1 monomer, 19 kDa protein
Histone H4 monomer, 11 kDa Xenopus laevis protein
Histone H3 full-length monomer, 15 kDa Xenopus laevis protein
|
| Buffer: |
50 mM citrate, 150 mM NaCl, 5 mM BME, 100% D2O, pH: 6.5 |
| Experiment: |
SANS
data collected at D22, Institut Laue-Langevin (ILL) on 2018 May 30
|
Histone chaperone exploits intrinsic disorder to switch acetylation specificity (Asf1-H3:H4-Rtt109-Vps75 protein complex, data for docking block selections)
Nat Commun 10(1):3435 (2019)
Danilenko N, Lercher L, Kirkpatrick J, Gabel F, Codutti L, Carlomagno T
|
| RgGuinier |
3.5 |
nm |
| Dmax |
11.0 |
nm |
|
|
UniProt ID: Q07794 (1-436) Histone acetyltransferase RTT109
UniProt ID: P32447 (1-169) Histone chaperone ASF1
UniProt ID: P62799 (1-103) Histone H4
UniProt ID: Q6PI79 (1-136) Histone H3 full-length
UniProt ID: P53853 (1-264) Vacuolar protein sorting-associated protein 75 full-length
|
|
|
|
| Sample: |
Histone acetyltransferase RTT109 monomer, 50 kDa Saccharomyces cerevisiae protein
Histone chaperone ASF1 monomer, 19 kDa protein
Histone H4 monomer, 11 kDa Xenopus laevis protein
Histone H3 full-length monomer, 15 kDa Xenopus laevis protein
Vacuolar protein sorting-associated protein 75 full-length dimer, 61 kDa Saccharomyces cerevisiae protein
|
| Buffer: |
50 mM citrate, 150 mM NaCl, 5 mM BME, 100% D2O, pH: 6.5 |
| Experiment: |
SANS
data collected at D22, Institut Laue-Langevin (ILL) on 2016 Jun 9
|
Histone chaperone exploits intrinsic disorder to switch acetylation specificity (Asf1-H3:H4-Rtt109-Vps75 protein complex, data for docking block selections)
Nat Commun 10(1):3435 (2019)
Danilenko N, Lercher L, Kirkpatrick J, Gabel F, Codutti L, Carlomagno T
|
| RgGuinier |
3.5 |
nm |
| Dmax |
11.5 |
nm |
|
|
UniProt ID: Q07794 (1-436) Histone acetyltransferase RTT109
UniProt ID: P32447 (1-169) Histone chaperone ASF1
UniProt ID: P62799 (1-103) Histone H4
UniProt ID: Q6PI79 (1-136) Histone H3 full-length
UniProt ID: P53853 (1-264) Vacuolar protein sorting-associated protein 75 full-length
|
|
|
|
| Sample: |
Histone acetyltransferase RTT109 monomer, 50 kDa Saccharomyces cerevisiae protein
Histone chaperone ASF1 monomer, 19 kDa protein
Histone H4 monomer, 11 kDa Xenopus laevis protein
Histone H3 full-length monomer, 15 kDa Xenopus laevis protein
Vacuolar protein sorting-associated protein 75 full-length dimer, 61 kDa Saccharomyces cerevisiae protein
|
| Buffer: |
50 mM citrate, 150 mM NaCl, 5 mM BME, 42% D2O, pH: 6.5 |
| Experiment: |
SANS
data collected at D22, Institut Laue-Langevin (ILL) on 2016 Jun 9
|
Histone chaperone exploits intrinsic disorder to switch acetylation specificity (Asf1-H3:H4-Rtt109-Vps75 protein complex, data for docking block selections)
Nat Commun 10(1):3435 (2019)
Danilenko N, Lercher L, Kirkpatrick J, Gabel F, Codutti L, Carlomagno T
|
| RgGuinier |
3.1 |
nm |
| Dmax |
9.5 |
nm |
|
|
UniProt ID: Q92133 (None-None) Histone H3
UniProt ID: P62799 (None-None) Histone H4
UniProt ID: P06897 (None-None) Histone H2A type 1
UniProt ID: Q92130 (None-None) Histone H2B
UniProt ID: None (None-None) Non-linker Ended Trinucleosome DNA
|
|
|
|
| Sample: |
Histone H3 monomer, 15 kDa Xenopus laevis protein
Histone H4 monomer, 11 kDa Xenopus laevis protein
Histone H2A type 1 monomer, 14 kDa Xenopus laevis protein
Histone H2B monomer, 14 kDa Xenopus laevis protein
Non-linker Ended Trinucleosome DNA monomer, 172 kDa DNA
|
| Buffer: |
20 mM Tris 150 mM NaCl 1 mM EDTA 1 mM DTT 50% w/v sucrose, pH: 7.5 |
| Experiment: |
SAXS
data collected at G1, Cornell High Energy Synchrotron Source (CHESS) on 2016 Mar 11
|
Solution structure(s) of trinucleosomes from contrast variation SAXS
Nucleic Acids Research (2021)
Mauney A, Muthurajan U, Luger K, Pollack L
|
| RgGuinier |
12.9 |
nm |
| Dmax |
41.8 |
nm |
|
|
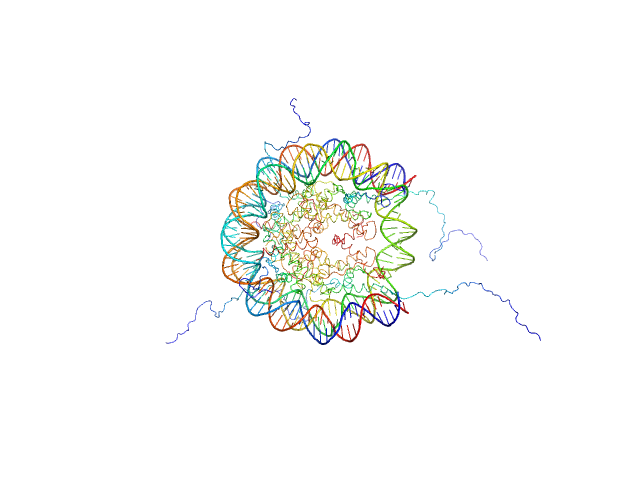
UniProt ID: P84233 (1-135) Histone H3.2
UniProt ID: P62799 (1-103) Histone H4
UniProt ID: Q6AZJ8 (1-130) Histone H2A
UniProt ID: P02281 (1-126) Histone H2B 1.1
UniProt ID: None (None-None) Widom 601 DNA sequence
|
|
|
|
| Sample: |
Histone H3.2 dimer, 31 kDa Xenopus laevis protein
Histone H4 dimer, 23 kDa Xenopus laevis protein
Histone H2A dimer, 28 kDa Xenopus laevis protein
Histone H2B 1.1 dimer, 28 kDa Xenopus laevis protein
Widom 601 DNA sequence dimer, 89 kDa Escherichia coli DNA
|
| Buffer: |
20 mM Tris-HCl , 50 mM KCl, 1 mM DTT, pH: 8 |
| Experiment: |
SAXS
data collected at SWING, SOLEIL on 2022 Dec 2
|
BCL7 proteins, novel subunits of the mammalian SWI/SNF complex, bind the nucleosome core particle
Asgar Abbas kazrani
|
| RgGuinier |
4.3 |
nm |
| Dmax |
12.3 |
nm |
| VolumePorod |
350 |
nm3 |
|
|