UniProt ID: Q4VC05-2 (1-231) Isoform 2 of B-cell CLL/lymphoma 7 protein family member A
UniProt ID: P84233 (1-135) Histone H3.2
UniProt ID: P62799 (1-103) Histone H4
UniProt ID: Q6AZJ8 (1-130) Histone H2A
UniProt ID: P02281 (1-126) Histone H2B 1.1
UniProt ID: None (None-None) Widom 601 DNA sequence
|
|
|
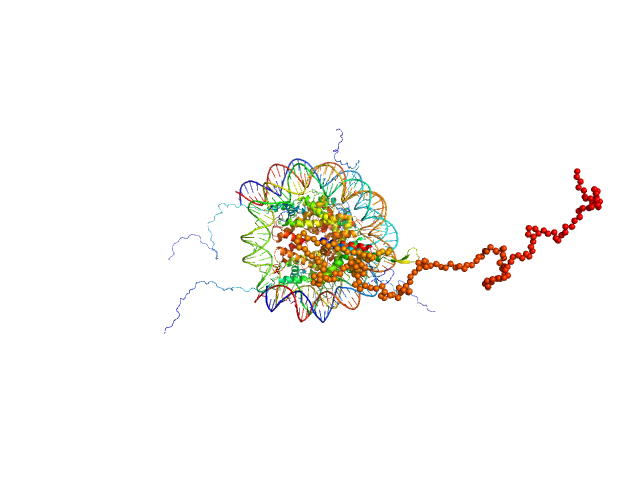
|
| Sample: |
Isoform 2 of B-cell CLL/lymphoma 7 protein family member A monomer, 25 kDa Homo sapiens protein
Histone H3.2 dimer, 31 kDa Xenopus laevis protein
Histone H4 dimer, 23 kDa Xenopus laevis protein
Histone H2A dimer, 28 kDa Xenopus laevis protein
Histone H2B 1.1 dimer, 28 kDa Xenopus laevis protein
Widom 601 DNA sequence dimer, 89 kDa Escherichia coli DNA
|
| Buffer: |
20 mM Tris-HCl , 50 mM KCl, 1 mM DTT, pH: 8 |
| Experiment: |
SAXS
data collected at SWING, SOLEIL on 2022 Dec 2
|
BCL7 proteins, novel subunits of the mammalian SWI/SNF complex, bind the nucleosome core particle
Asgar Abbas kazrani
|
| RgGuinier |
4.7 |
nm |
| Dmax |
24.4 |
nm |
| VolumePorod |
400 |
nm3 |
|