|
Synchrotron SAXS data from solutions of truncated human βB2-crystallin in 25 mM sodium phosphate, 5 mM DTT, 1 mM EDTA, pH 6.5, were collected on the 12ID-B SAXS/WAXS beam line at the Advanced Photon Source (APS, Argonne, IL, USA) using a Pilatus 2M detector at a sample-detector distance of 2 m and at a wavelength of λ = 0.08856 nm (I(s) vs s, where s = 4πsinθ/λ and 2θ is the scattering angle). Data were acquired over 30 successive 0.400 second frames from a sample at 1.75 mg/ml. The data were normalized to the intensity of the transmitted beam and radially averaged. The scattering of the radially-averaged solvent-blank was subtracted to produce the scattering profile displayed in this entry.
Cell temperature = UNKNOWN. Storage temperature = UNKNOWN
|
|
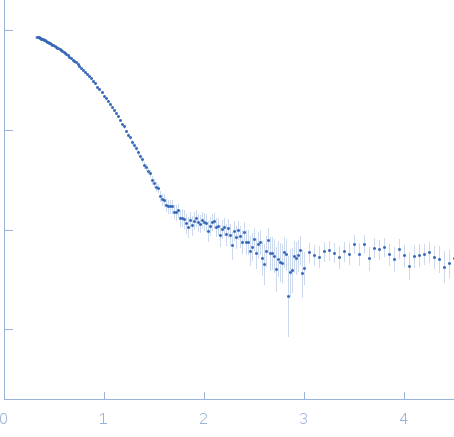 s, nm-1
s, nm-1