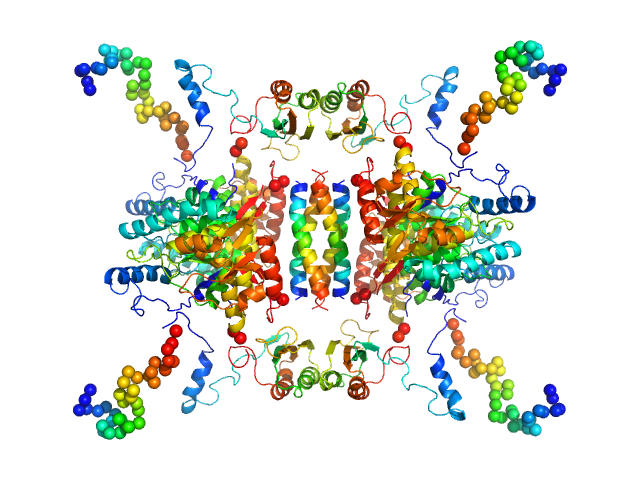
|
X-ray synchrotron radiation scattering data from solutions of tyrosine hydroxylase, isoform 1 in 20 mM Na-HEPES, 200 mM NaCl, pH 7.0 were collected on the P12 beam line of Petra-III (Hamburg, Germany) using a Pilatus 2M detector (I(s) vs s; s = 4π sin θ/λ, where 2θ is the scattering angle and λ=0.124 nm). Different solute concentrations in the range 1.0-2.0 mg/ml were measured using an exposure time of 1 s (recorded as 20 x 0.050 s frames). The data were normalized to the intensity of the transmitted beam and radially averaged and the scattering from the matched solvent-blank was subtracted. The data presented here are merged SAXS data derived from the solute concentration series.
|
|
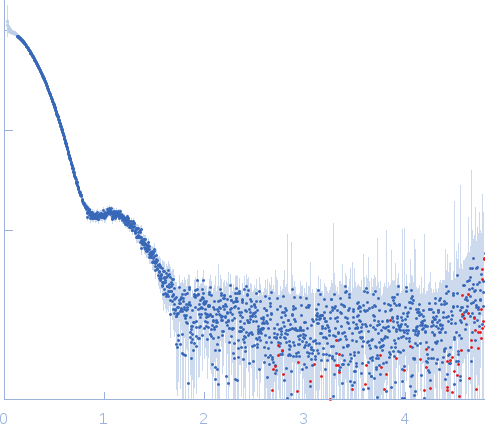 s, nm-1
s, nm-1