|
Synchrotron SAXS data from solutions of the citrate-binding PAS domain from the sensor histidine kinase, CitA, fused to lipase EstA in 10 mM glycine buffer, 10 mM NaCl, pH 10 were collected on the BM29 beam line at the ESRF (Grenoble, France) using a Pilatus 1M detector at a sample-detector distance of 2.8 m and at a wavelength of λ = 0.09919 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 4.98 mg/ml was measured at 20°C. 10 successive 1 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
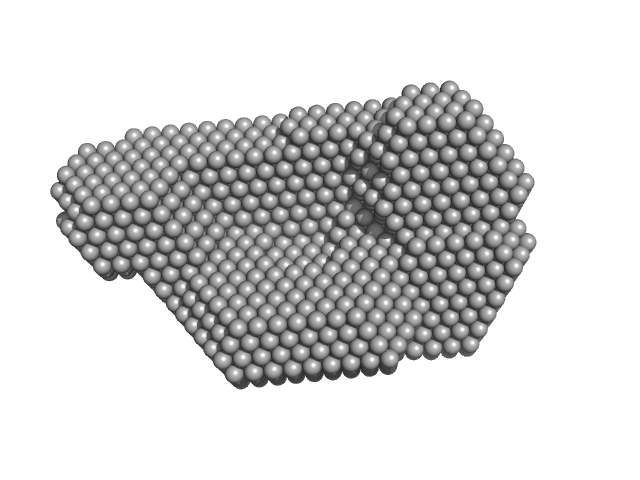
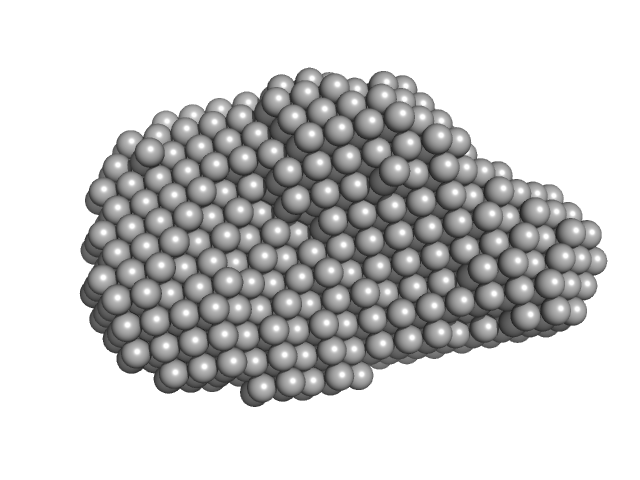
The protein is a fusion of: i) CitAP - the PAS domain from Sensor histidine kinase, CitA, from Klebsiella pneumoniae; UniProt: P52687 (Amino acid range 45-177); ii) BsLA - the Lipase EstA from Bacillus subtilis 168; UniProt: I6V559 (Amino acid range 32-212) and; iii) A linker connecting CitAP with EstA - derived from the blue light photoreceptor, YtvA, from Bacillus subtilis 168; UniProt: O34627 (Amino acid range 132-147).
|
|
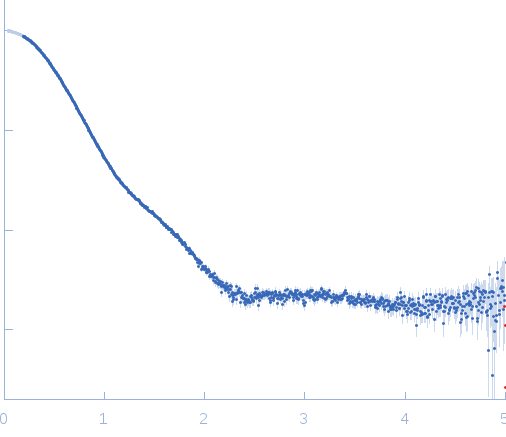 s, nm-1
s, nm-1