|
Synchrotron SAXS data from solutions of the XthA, sliding beta clamp, DNA ligase-A tricomplex with DNA substrate in 50 mM Tris pH 8.0, 200 mM NaCl , 2 mM β-mercaptoethanol, were collected on the BM29 beam line at the ESRF storage ring (Grenoble, France) using a Pilatus 1M detector at a sample-detector distance of 2.9 m and at a wavelength of λ = 0.0992 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 2.50 mg/ml was measured at 10°C. 10 successive 1 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
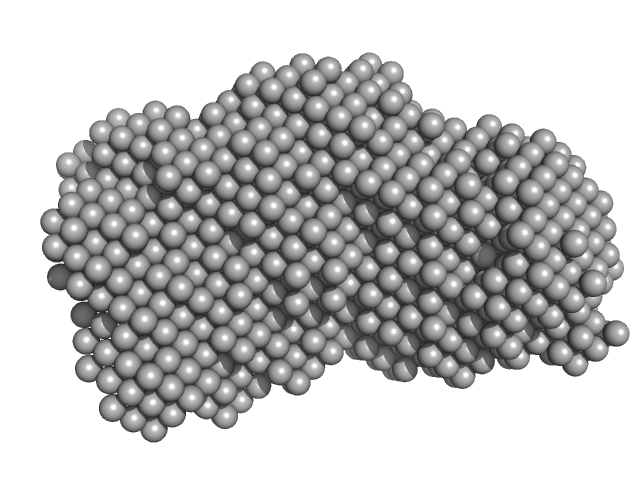
The presented model shows the spatial alignment of several individually calculated models and does not fit the SAXS data. A corresponding individual model fit (individual model not provided for this entry) is shown. The sample is severely aggregated.
|
|
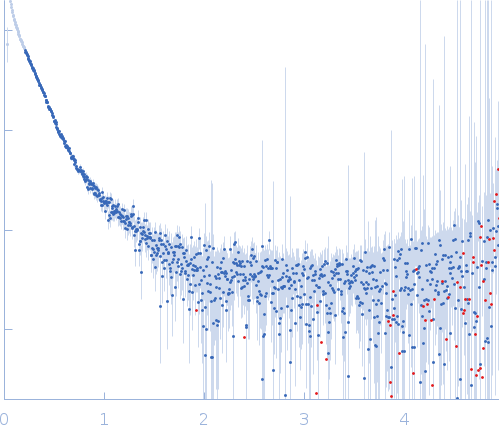 s, nm-1
s, nm-1