|
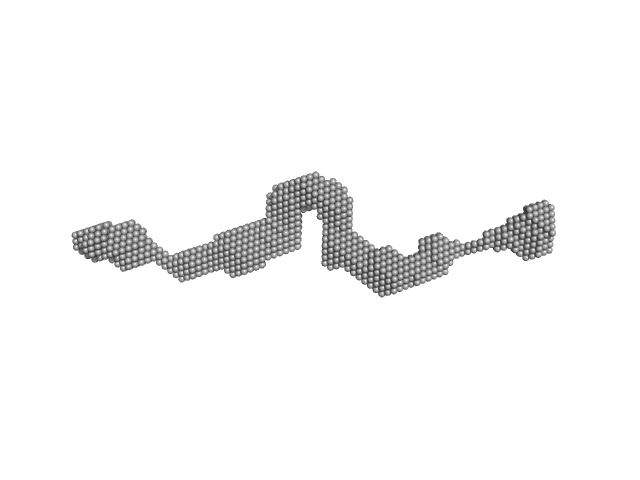
SANS data from solutions of the R11-15 human dystrophin fragment with zwitterionic phospholipid bicelles in 20 mM Tris-d11, 150 mM NaCl, 0.1 mM EDTA-d16, in 100% v/v D2O, pH 7.1 were collected on the D22 beam line at the ILL (Grenoble, France) using a 3He multidetector 128 linear sensitive Reuter-Stokes detector at a wavelength of λ = 0.6 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 5.60 mg/ml was measured at 22°C. One 1200 second frame was collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
Data were collected at two sample detector positions: 1) 1.4 m for 5 min using a neutron wavelength of 0.6 nm and; 2) 8 m for 20 min using a neutron wavelength of 0.6 nm. Both datasets were recorded on the same sample at 5.6 mg/mL, measured at 22 °C.
|
|
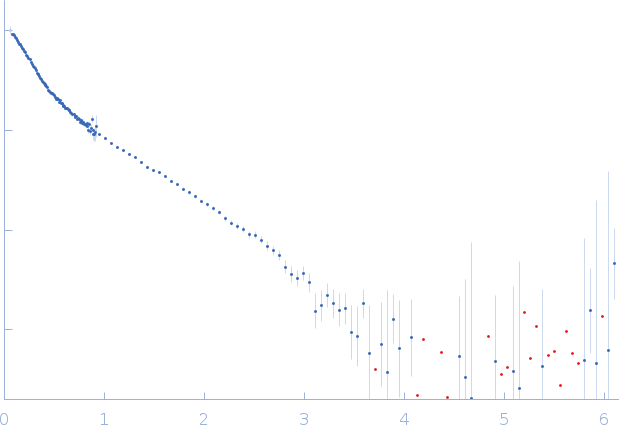 s, nm-1
s, nm-1