|
SAXS data were collected in the bioSAXS beamline B21, at Diamond Light Source, Harwell, United Kingdom. For the SYT1SMP2C2A, 45 μl of sample with a concentration of 6.6 mg/ml, were injected onto a Superdex 200 increase 3.2/300 column at 20°C using 20 mM Tris pH 8, 100 mM NaCl, 5% glycerol, 2 mM DTT as the running buffer. The output flow from the Agilent HPLC was directed through a 1.6 mm diameter quartz capillary cell held in vacuum. The flow rate was set to 0.08 mL/min and 620 frames (with an exposure time of 3 s) were collected using a PILATUS 2M (Dectris, Switzerland) detector at the distance of 4.014 m from the sample. The collected two-dimensional images were corrected for variations in beam current, normalized for exposure time and processed into one- dimensional scattering curves using GDA and the DAWN software (Diamond Light Source, UK). The background was manually subtracted using the program ScÅtter and CHROMIXS (ATSAS).
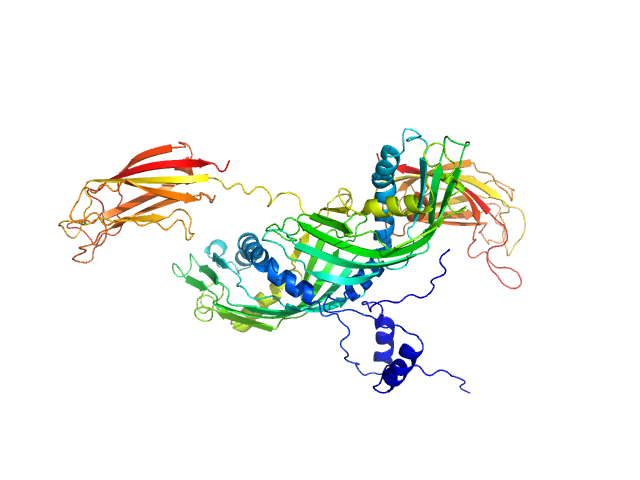
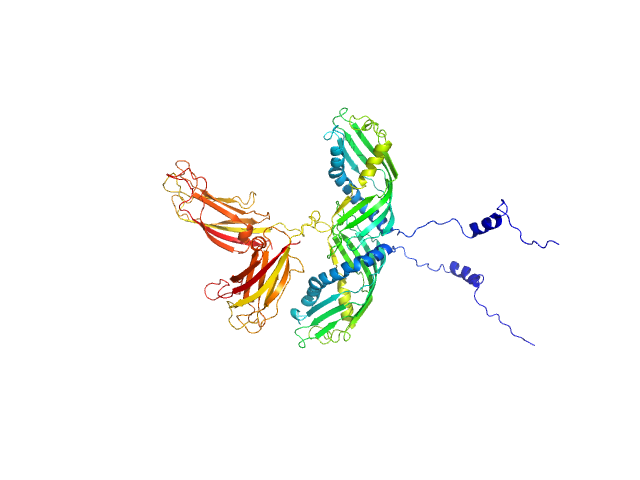
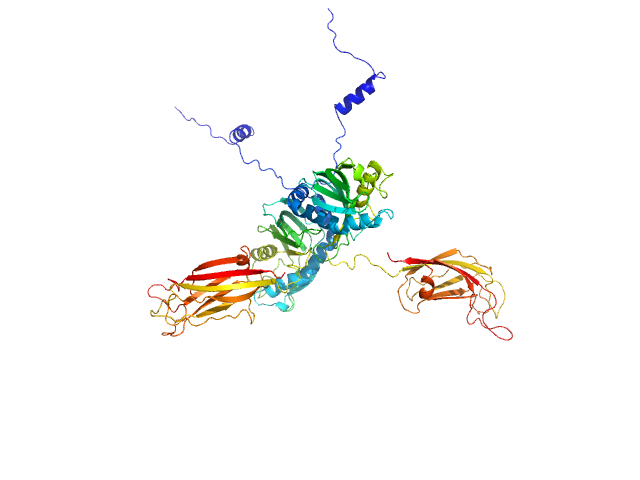
To build a model of the protein in solution we used the high-resolution atomic structure of SYT1C2A and a model based on the atomic structure of the SMP domain from E-Syt2 and used the SAXS experimental data for a low resolution rigid-body modeling (CORAL). In addition, we model-built residues N34 to K67 and the linker between the SMP and C2A domain that were not present in the initial model. We repeated this procedure several times fixing different linker lengths between SMP and SYT1C2A to select those models that adjusted better to the experimental data. However, the agreement with the SAXS experimental curves of the models obtained was similar. This indicates that SMPC2A does not display a unique structure in solution.
|
|
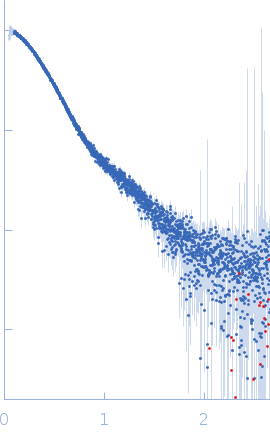 s, nm-1
s, nm-1