|
Synchrotron SAXS
data from solutions of
Serine/threonine-protein phosphatase (PP1) bound to Protein phosphatase 1 (PTG) and cyclodextrin
in
50 mM Tris pH 8.0, 0.5 M NaCl, 10% glycerol, 1 mM DTT, pH 8
were collected
on the
BM29 beam line
at the ESRF storage ring
(Grenoble, France)
using a Pilatus3 2M detector
at a wavelength of λ = 0.09 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
In-line size-exclusion chromatography (SEC) SAS was employed. The SEC parameters were as follows: A 50.00 μl sample
at 6.5 mg/ml was injected at a 0.30 ml/min flow rate
onto a Agilent AdvanceBio SEC 130Å, 4.6 x 50 mm column
.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
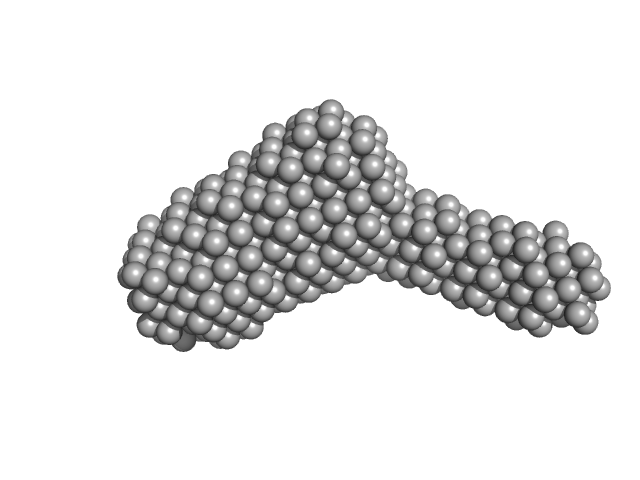
I(s) versus s experimental SAXS profile for PTG-PP1 complex bound to 1 molecule of cyclodextrin. Data were collected at ESRF BM29 using SEC-SAXS approach. In the SEC-SAXS chromatogram, frames in regions of stable Rg were selected with CHROMIXS and averaged using PRIMUS to yield a single averaged frame per protein sample. Sample was loaded on an AdvanceBio SEC 130 Å (4.6 x 50 mm) column (Agilent) via a high-performance liquid chromatography device (HPLC, Shimadzu) attached directly to the sample-inlet valve of the BM29 sample changer. Protein phosphatase that associates with over 200 regulatory proteins to form highly specific holoenzymes which dephosphorylate hundreds of biological targets. Protein phosphatase 1 (PP1) is essential for cell division, and participates in the regulation of glycogen metabolism, muscle contractility and protein synthesis. The Protein phosphatase 1 regulatory subunit 3C (PTG) acts as a glycogen-targeting subunit for PP1 and regulates its activity.
|
|
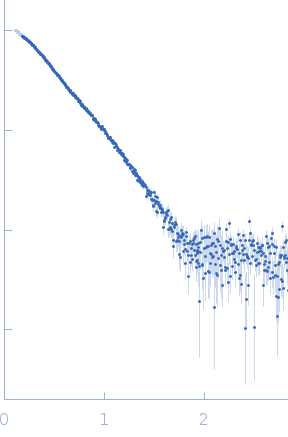 s, nm-1
s, nm-1