|
SANS data from solutions of His-tagged L-methionine gamma-lyase from Clostridium tetani in PBS-D2O (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4 (D2O buffer), pD 7.4) performed on the YuMO SANS TOF spectrometer (IBR-2: Frank Laboratory of Neutron Physics, Joint Institute for Nuclear Research; Dubna, Russian Federation) with a two He3-fulfilled, 8 independent wire detector system (sample-detector distances of 4.5 m and 12.97 m). The wavelengths used are from 0.05 to 0.8 nm, the contributions of which are separated using time-of-flight technology. One solute concentration of 10.00 mg/ml was measured at 20°C. 9000 successive 0.207 second frames were collected.
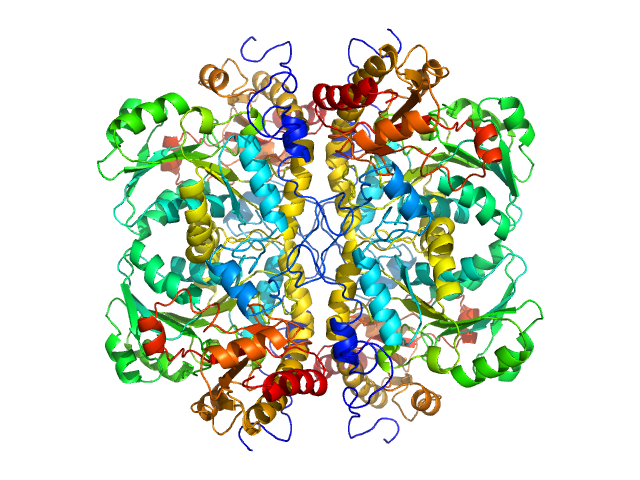
The protein of study is His-tagged L methionine gamma lyase from Clostridium tetani. The volume fractions of tetramers and octamers in solution are 37% and 63%, respectively.
|
|
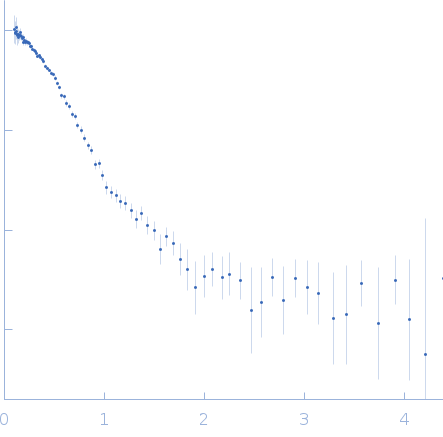 s, nm-1
s, nm-1