|
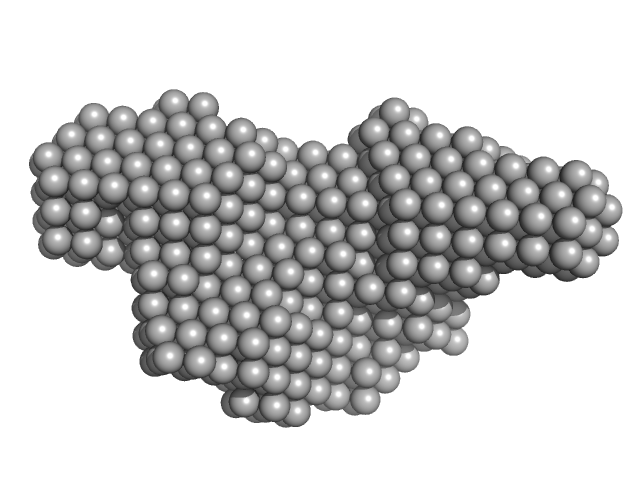
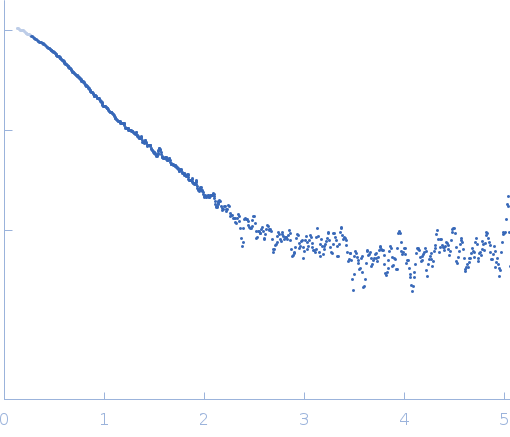
Synchrotron SAXS data from solutions of Human DjC20/DnaJC20/HscB iron-sulfur cluster co-chaperone protein in 25 mM Tris-HCl, 50 mM NaCl, 5 mM KCl, 2 mM β-mercaptoethanol, pH 7.5 were collected on the SAXS1 beam line at the Brazilian Synchrotron Light Laboratory (LNLS, Campinas, São Paulo, Brazil) using a Pilatus 300K detector at a sample-detector distance of 1 m and at a wavelength of λ = 0.1488 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). Solute concentrations at 1.2 mg/mL, 1.3 mg/mL, and 2.5 mg/mL were measured at 20°C (300 second exposures). The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted. The data from the concentration series were processed and then merged to a single curve using PRIMUS and DATMERGE softwares. GNOM software was used to generate the pair-distance distribution function of DjC20. Ten ab initio dummy atom models were generated using DAMMIF and averaged by DAMAVER programs. The averaged model was refined using DAMMIN software.
|
|
 s, nm-1
s, nm-1